| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 682-694
Accumulation of CD56+ CD16- Natural Killer Cells in Response to Preoperative Chemotherapy for Breast Cancer
Ryungsa Kima, e, Ami Kawaia, Megumi Wakisakaa, Mika Shimoyamaa, Naomi Yasudaa, Mitsuya Itob, Takanori Kinc, Koji Arihirod
aDepartment of Breast Surgery, Hiroshima Mark Clinic, Hiroshima, Japan
bDepartment of Breast Surgery, Hiroshima City Hospital, Hiroshima, Japan
cDepartment of Breast and Endocrine Surgery, Osaka University Graduate School of Medicine, Osaka, Japan
dDepartment of Anatomical Pathology, Hiroshima University Hospital, Hiroshima, Japan
eCorresponding Author: Ryungsa Kim, Department of Breast Surgery, Hiroshima Mark Clinic, Naka-ku, Hiroshima 730-0051, Japan
Manuscript submitted April 23, 2024, accepted May 28, 2024, published online July 5, 2024
Short title: CD56+ CD16- NK Cells and Chemotherapy Response
doi: https://doi.org/10.14740/wjon1885
| Abstract | ▴Top |
Background: The activation of the antitumor immune responses of T cells and natural killer (NK) cells is important to induce breast tumor shrinkage via preoperative chemotherapy. We evaluated how antitumor immune responses contribute to the effects of such therapy.
Methods: Forty-three patients with stages I - IV breast cancer who underwent surgery between August 2018 and Jun 2023 after preoperative chemotherapy were enrolled. Peripheral natural killer (pNK) cell activity was assessed by 51Cr-release assay, and the counts and percentages of CD4+, CD8+, and NK cells and their subsets in peripheral blood were measured before and after chemotherapy by two-color flow cytometry. Associations of cell population changes with chemotherapy responses were analyzed.
Results: On univariate analysis, relative to grade (G) ≤ 1 effects, G ≥ 2 therapeutic effects were associated significantly with human epidermal growth factor receptor 2 (HER-2)+ breast cancer (P = 0.024) and post-chemotherapy CD56+ CD16- NK cell accumulation (8.4% vs. 5.5%, P = 0.042), and tended to be associated with increased pre-chemotherapy CD56+ CD16- NK cell percentages (5.4% vs. 3.3%, P = 0.054) and pNK cell activity (42.0% vs. 34.5%, P = 0.057). The accumulation and increased percentage of CD56+ CD16- NK cells in patients with G ≥ 2 effects were not associated with changes in pNK cell activity or the disappearance of axillary lymph-node metastases. On multivariate analysis, G ≥ 2 therapeutic effects tended to be associated with higher pre-chemotherapy pNK levels (odds ratio = 0.96; 95% confidence interval: 0.921 - 1.002; P = 0.067).
Conclusions: The accumulation of the immunoregulatory CD56+ CD16- NK cell subset in the peripheral blood before and after chemotherapy may lead to the production of cytokines that induce an antitumor immune response. Activation of the immune response mediated by CD56+ CD16- pNK cells after chemotherapy and their high counts before chemotherapy may contribute to the improvement of therapeutic effects against breast cancer.
Keywords: Breast cancer; Preoperative chemotherapy; CD56+ CD16-; Natural killer cell; Therapeutic effect
| Introduction | ▴Top |
Antitumor immunity plays an important role in the efficacy of cancer chemotherapy [1]. Among patients with triple-negative (TN) and human epidermal growth factor receptor 2 (HER-2)+ breast cancer, pathological complete response (pCR) and survival rates are higher than those with lower tumor-infiltrating lymphocyte (TIL) levels prior to preoperative chemotherapy [2-4]. In the presence of high TIL levels, the pCR rate is lower for hormone receptor (HR)+ HER-2- luminal (L) breast cancer than for TN or HER-2+ breast cancer [5]. However, a low pCR rate for L-type breast cancer is not necessarily associated with a poor prognosis, as this prognosis depends on the sensitivity to endocrine therapy after surgical treatment [6]. Antitumor immunity includes innate and adaptive immune responses by natural killer (NK) cells and cytotoxic T lymphocytes (CTLs); the activation of NK and T cells by preoperative chemotherapy contributes to primary breast tumor shrinkage and therapeutic efficacy [7-10]. Increases in NK and CD8 T cells and decreases in immunosuppressive factors such as regulatory T cells (Tregs), vascular endothelial growth factor (VEGF), and cytotoxic T lymphocyte antigen-4 (CTLA-4) in the tumor microenvironment have been reported [9].
Increased peripheral natural killer (pNK) cell activity before and after preoperative chemotherapy and increased NK cell concentrations in primary tumors contribute to the improvement of breast cancer treatment efficacy [8, 9, 11]. NK cells exert an innate immune response that is independent of the major histocompatibility complex (MHC) and induces the apoptotic death of cancer cells secreted by perforin and granzyme B. Thus, they have the important role of killing cancer cells that lack MHC I markers and are not detected and destroyed by other immune cells such as CTLs [12].
Two NK cell subsets with distinct phenotypic properties are usually identified by the surface markers CD56 and CD16 (FcγR III) in the absence of CD3: CD56bright or + CD16- (10-15% of pNK populations in healthy individuals) and CD56dim or - CD16+ (90-95%) [13, 14]. The CD56+ subset has an immunomodulatory function, producing cytokines such as interferon gamma (IFN-γ) and transforming growth factor beta (TGF-β), and the CD56- subset has a cytotoxic function, killing cancer cells with low cytokine levels [15]. These subsets can be classified further into four functionally and phenotypically distinct subsets: CD56+ CD16-, CD56- CD16-, CD56- CD16+, and CD56+ CD16+, whose distributions at tumor sites may depend on the tumor microenvironment [16-18]. CD56+ CD16- NK cells are proliferative, immature immune regulatory cells that can interact with adjacent immunocompetent cells in lymphoid tissue [19]. In contrast, CD56- CD16+ NK cells reach inflammatory sites and exert cytotoxic functions to promote immune responses [20, 21]. CD56+ CD16+ NK cells sometimes contain CD56+ CD16- subsets and undergo intermediate maturation, leading to the development of CD56- CD16+ NK cells. CD56- CD16- NK cells, which are more abundant at breast tumor sites, have fewer activation receptors and cytotoxic molecules.
In the tumor microenvironment, tumor-derived soluble factors such as TGF-β, prostaglandin E2, and indoleamine 2,3-dioxygenase are responsible for changes in NK cell phenotypes and functions [22]. Immunosuppressive immune cells such as M2-macrophages, myeloid-derived suppressor cells, and Tregs influence NK cell activity by releasing these soluble factors [22]. In particular, the TGF-β concentration was shown to correlate negatively with molecules associated with NK cell cytotoxicity; TGF-β promotes the conversion of CD56+ CD16+ NK cells to decidual-like CD56+ CD16- NK cells, a tumor-infiltrating NK cell phenotype involved in pro-angiogenic activity [17, 23].
Our previous studies have shown that increased pNK cell activity, together with the activation of CD4, CD8, and NK cells in the tumor microenvironment, is important for the achievement of preoperative chemotherapy efficacy in patients with breast cancer [8, 9, 11]. In the present study, we focused further on pNK cell activity, pNK subset distributions, and T cell percentages in the peripheral blood of different populations of patients with breast cancer before and after preoperative chemotherapy. The results provide further insight into the contributions of antitumor immune responses activated by anticancer agents to the efficacy of preoperative chemotherapy in patients with breast cancer.
| Materials and Methods | ▴Top |
Study design
Patients with stages I - IV breast cancer who received preoperative chemotherapy between August 2018 and June 2023 were enrolled in this cohort study. Blood samples were drawn before and after chemotherapy to analyze pNK cell activity and to measure the percentages of CD4, CD8, and NK cells and their subsets. This retrospective cohort study was conducted in accordance with the Declaration of Helsinki. The Ethics Committee of the Hiroshima Mark Clinic approved this study (number: HMC-05), and all specimens were collected with patients’ informed consent.
Patient eligibility
Eligible patients were women (menopausal status was not a criterion) aged < 80 years with an Eastern Cooperative Oncology Group performance status of 0; white blood cell counts ≥ 3,000/mm3; platelet counts ≥ 100,000/mm3; and normal bilirubin, aspartate aminotransferase/alanine aminotransferase, alkaline phosphatase, and creatine levels. Patients with active cardiac disease, pregnant patients, those treated previously for breast cancer and/or with anthracycline for malignancy, and patients receiving concurrent sex hormone therapy were excluded. Patients with stage IV disease with multiple lung and/or liver metastases were also excluded. Breast cancer stages were determined according to the tumor-lymph node-metastasis classification scheme recommended by the Union for International Cancer Control [24]. Axillary lymph node (ALN) metastasis (Ax+) was confirmed by fine-needle aspiration biopsy. For stage IV cases, palliative surgery was performed for the effective local control of primary breast cancer.
Clinicopathological factors
For the comparison of preoperative chemotherapy effects, data on clinicopathological factors such as the tumor stage, subtype, histology, treatment regimen, nuclear grade, and Ki-67 positivity were collected. Data reflecting antitumor immune responses (e.g., NK and T cell percentages in peripheral blood) were also collected.
Assessment of pathological and therapeutic effects of preoperative chemotherapy
The pathological and therapeutic effects of preoperative chemotherapy were evaluated according to the histopathological criteria of the Japanese Breast Cancer Society [25, 26]. The pathological responses of intramammary lesions were graded as follows: grade 0 (G0), minimal or no cancer cell change; grade 1a (G1a), mild cancer cell changes or marked changes in less than one-third of cancer cells; grade 1b (G1b), marked changes in more than one-third but less than two-thirds of cancer cells; grade 2a (G2a), marked changes in more than two-thirds of cancer cells; grade 2b (G2b), disappearance of almost all cancer cells; and grade 3 (G3), apparent disappearance of all cancer cells. Treatment grades were determined according to ductal and/or ALN involvement. The resolution of lymph-node metastasis was noted when it occurred. Because the axillary status has been suggested to be a better prognostic factor than the primary tumor responsiveness to preoperative chemotherapy, the disappearance of invasive cancer cells from all breast tissues and ALNs was regarded as complete response (ypT0/is N0) [27, 28]. The disappearance of Ax+ was confirmed by the pathological analysis of surgically dissected ALNs.
Measurement of pNK cell activity
SRL, Inc. (Tokyo, Japan) measured NK cell activity in peripheral blood samples taken before and 3 - 4 weeks after chemotherapy using a chromium release assay, as described previously [8, 9, 11]. Briefly, pNK cells were assayed for cytotoxic activity against 51Cr-labeled target (K562) cells. After lymphocyte isolation, effector and target cells were adjusted to a 20:1 ratio, plated, and incubated at 37 °C for 3.5 h under CO2. The cells were then centrifuged, and supernatants were collected and counted in a gamma counter. pNK cell activity was calculated as experimental group release - background release/maximal release - background release.
Measurement of CD4, CD8, and NK cells and their subsets in peripheral blood
SRL, Inc. determined counts and percentages of CD4, CD8, and NK cells and their subsets in peripheral blood collected before and after chemotherapy using two-color flow cytometry. In brief, two monoclonal antibody pairs (T4/T8 (Beckman Coulter, Brea, CA, USA) and CD16/CD56 (BD Biosciences, Franklin Lakes, NJ, USA/Beckman Coulter)) conjugated with fluorescein isothiocyanate and phycoerythrin were used. The samples and monoclonal antibodies were dispended, and specimen aliquots were adjusted to 50 µL and incubated for 20 min at 4 °C in the dark. Then, lysing reagent was added with mixing, and the specimens were left at room temperature for 10 min. Centrifugal washing was then performed, and supernatants were removed. Phosphate-buffered saline was added, followed by centrifugation, supernatant removal, and analysis by flow cytometry (FACS Canto II; BD Biosciences). The following gating strategy was used for NK cell subsets. Using a histogram with a forward scatter (FSC)/side scatter (SSC) parameter set, gating was performed on a population of FSC-low and SSC-low (lymphocyte) areas. The gated lymphocyte population was subjected to two-color analysis of CD56/CD16 as a population. The distributions of CD56 (phycoerythrin) and CD16 (fluorescein isothiocyanate) staining were examined for each label; two-color parameter dot plots of CD56/CD16 on the y and x axes were expanded to observe the distributions of CD56+ CD16-, CD56+ CD16+, CD56- CD16+, and CD56- CD16- cells. Positivity rates of these cells relative to the total population were then calculated.
Statistical analysis
All data were analyzed using Statcel 4 software (OMS Publishing Inc., Tokyo, Japan). Continuous and independent variables were analyzed using the Mann-Whitney test. Categorical variables were analyzed using the Chi-squared test and Fisher’s exact test. Univariate and multivariate analyses were performed to evaluate associations of dependent variables (therapeutic effects) with independent variables (clinicopathological factors, pNK cell activity, NK cell subsets (CD56+ CD16-, CD56- CD16-, CD56- CD16+, CD56+ CD16+), CD4 and CD8 concentrations, and the CD4/CD8 ratio). Odds ratios (ORs) with 95% confidence intervals (CIs) are reported. P values < 0.05 were considered to be significant.
| Results | ▴Top |
Patient and treatment characteristics
In total, 43 patients (median age: 55 years; range: 31 - 76 years) with stages I (n = 1), II (n = 29), III (n = 8), and IV (n = 5) breast cancer were included in this study. The clinical characteristics of the stage-IV cases were T4bN3M1 (bone/liver), T4bN3M1 (bone), T3N2M1 (bone), T3N0M1 (bone), and T1N1M1 (bone), respectively. The tumor subtypes were L (n = 29), HER-2+ (n = 7), and TN (n = 7). The histological types were invasive ductal carcinoma, not otherwise specified (n = 38); invasive lobular carcinoma (n = 2); and other (n = 3). The treatment regimens included taxanes, epirubicine (E), cyclophosphamide (C), and trastuzumab (Tz) and/or pertuzumab (Pz). Thirty-eight patients were treated with EC + taxanes (six with Tz and five with Pz), one patient was treated with EC + dose-dense (dd) albumin-bound paclitaxel (nab-PTX) and ddEC + nab-PTX, two patients were treated with ddEC + ddnab-PTX, and one patient was treated with docetaxel + carboplatin + Pz + Tz followed by ddEC. Pz and Tz were used for HER-2+ tumors. Taxanes were given as nab-PTX, docetaxel, or paclitaxel. The pathological and therapeutic responses were G0 in two patients, G1a in eight patients, G1b in 10 patients, G2a in six patients, G2b in four patients, and G3 (complete) in 13 patients. The pCR rate was 18.6%.
Associations of clinicopathological factors with pathological effects
Relative to G ≤ 1 effects, G ≥ 2 therapeutic effects were associated significantly with HER-2+ breast cancer (P = 0.024), treatment with Tz (P = 0.008) and Pz (P = 0.017), high Ki-67+ rates (P = 0.036), and high pre-chemotherapy pNK cell activity (P = 0.057; Table 1, Fig. 1). Relative to Ax+, the disappearance of Ax+ was associated significantly with the treatment of HER-2+ breast cancer with Tz and/or Pz (P = 0.046), which yielded G ≥ 2 therapeutic effects, and higher pre-chemotherapy Ki-67+ rates (P = 0.040) (Table 2). HER-2+ breast cancer was more responded to targeted combination chemotherapy than are HR+ HER- and TN (any HR status) breast cancers. Relative to lower rates, higher Ki-67+ rates were associated with greater sensitivity to anticancer drugs. Pre-chemotherapy pNK cell activity was significantly lower among patients in whom this activity subsequently increased than among those in whom it decreased (P = 0.030), and the post-chemotherapy level was higher in the former group (P = 0.005) (Table 3). Differences in pNK cell activity between the decrease and increase groups were not associated with any clinicopathological factor in 41 patients, excluding two patients with no change in this activity (Table 3). These findings are consistent with our previous reports on different patient populations and suggest that increases in pNK activity after preoperative chemotherapy reflecting systemic immune activation contribute to the elimination of metastatic tumor cells via the local release of immunosuppressive factors in the tumor microenvironment [8].
 Click to view | Table 1. Univariate Associations of Pathological Responses With Clinicopathological Factors in 43 Patients With Breast Cancer Who Received Preoperative Chemotherapy |
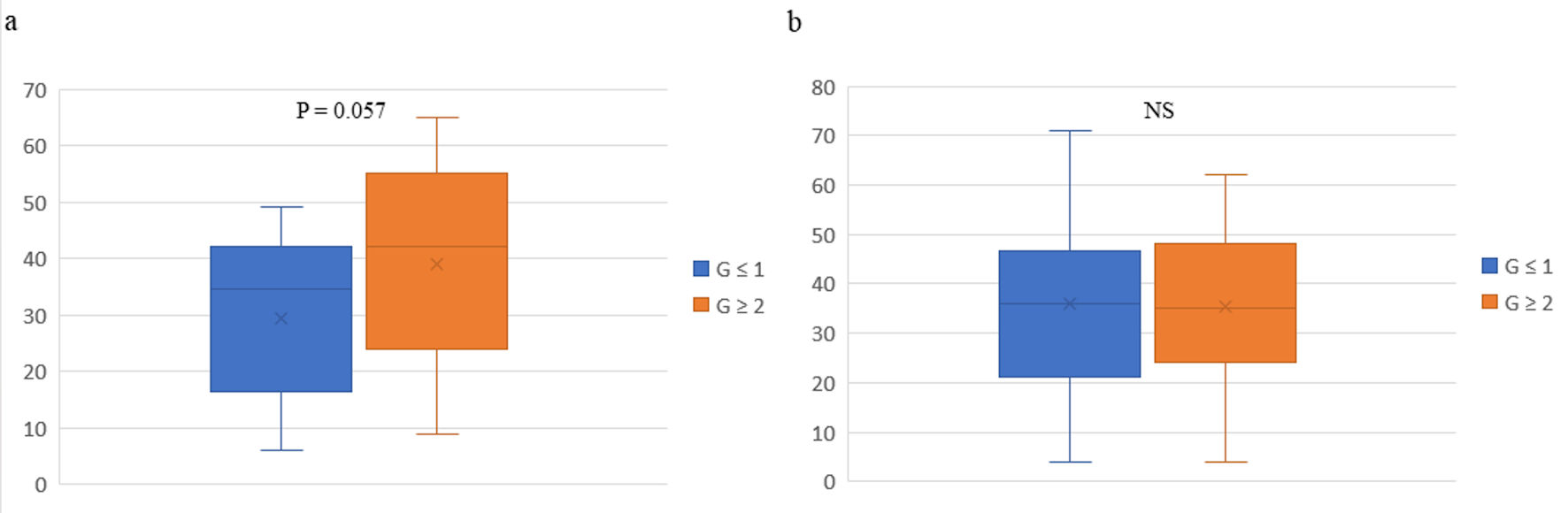 Click for large image | Figure 1. Box plots of pathological responses in primary tumors and peripheral natural killer cell activity before (a) and after (b) preoperative chemotherapy in 43 patients with breast cancer. The vertical axis represents peripheral natural killer cell activity. The box-plot analysis was performed using Excel software and individual data on pNK cell activity. The whiskers are error bars representing the minimum - maximum range, with the interquartile (25th - 75th percentile) range extended 1.5 times. The bottoms and tops of the boxes are the 25th and 75th percentiles, respectively, the lines in the boxes are the 50th percentiles (medians), and the Xs are the means. G: grade; NS: not significant. |
 Click to view | Table 2. Univariate Associations of Disappearance of Axillary Lymph Node Metastasis With Clinicopathological Factors in 26 Patients With Breast Cancer Who Received Preoperative Chemotherapy |
 Click to view | Table 3. Univariate Associations of pNK Cell Activity With Clinicopathological Factors in 41 Patients With Breast Cancer Who Received Preoperative Chemotherapy |
Associations of pNK cell subsets with pathological effects
Among pNK cell subsets, the median percentage of CD56- CD16- NK cells was highest, followed by those of CD56+ CD16+, CD56- CD16+, and CD56+ CD16- NK cells. These findings differ from previous reports that the most common pNK subset in patients with breast cancer is CD56dim CD16+, although the percentages of the other subsets are similar [16]. Relative to G ≤ 1 effects, G ≥ 2 therapeutic effects tended to be associated with increased pre-chemotherapy (P = 0.054) and post-chemotherapy (P = 0.042) percentages of CD56+ CD16- NK cells (Table 1, Fig. 2). No such association was observed with the percentages of other pNK cell subtypes, the percentages of CD4 or CD8 T cells, or the CD4/8 ratio (Table 1). These findings suggest that increases in CD56+ CD16- pNK cells before and after preoperative chemotherapy contribute to the activation of antitumor immunity, and thus the improvement of the therapeutic effect, in patients with breast cancer. Nevertheless, G ≥ 2 therapeutic effects tended to be associated only with higher pre-chemotherapy pNK levels in the multivariate regression analysis (OR = 0.96; 95% CI: 0.92 - 1.00; P = 0.067) (Table 4); they were not associated with higher pre- or post-chemotherapy percentages of CD56+ CD16- NK cells. These results suggest that increases in CD56+ CD16- NK cells contribute less to the systemic immune response that improves the therapeutic effect than do increases in pNK cell activity although an increase in CD56+ CD16- NK cells may affect pNK cell activity.
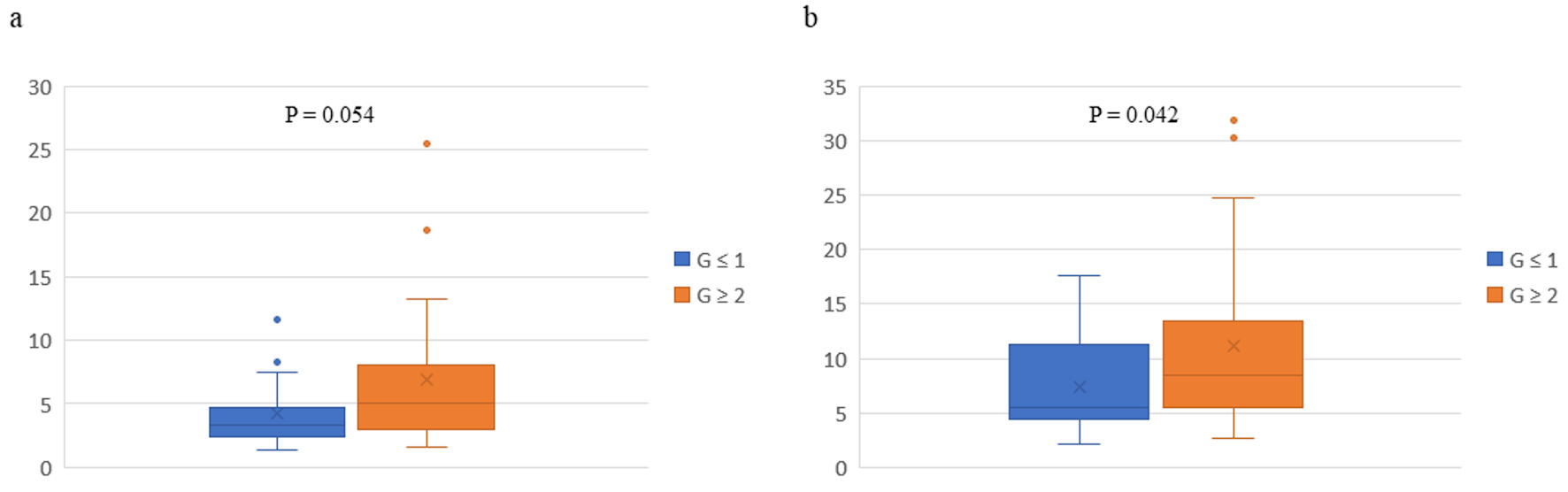 Click for large image | Figure 2. Box plots of pathological responses in primary tumors and percentages of CD56+ CD16- natural killer (NK) cells before (a) and after (b) preoperative chemotherapy in 43 patients with breast cancer. The vertical axis represents percentage of CD56+ CD16- NK cells. The whiskers are error bars representing the minimum - maximum range, with the interquartile (25th - 75th percentile) range extended 1.5 times. The bottoms and tops of the boxes are the 25th and 75th percentiles, respectively, the lines in the boxes are the 50th percentiles (medians), outliers are shown as closed circles, and the Xs are the means. G: grade. |
 Click to view | Table 4. Multivariate Associations of G2 and Better Therapeutic Effects With pNK Cell Activity Before Chemotherapy, CD56+ CD16- NK Cell Subset Before and After Chemotherapy in 43 Patients With Breast Cancer Who Received Preoperative Chemotherapy |
| Discussion | ▴Top |
In this study, patients who responded to preoperative chemotherapy tended to have higher percentages of CD56+ CD16- pNK cells before and after chemotherapy and more pNK cells derived from bone marrow before chemotherapy. CD56+ CD16- NK cells are less mature and poorly cytolytic compared with other NK cells, but they have an immunoregulatory phenotype, developing into CD56+ CD16+ and CD56- CD16+ NK cells that produce large quantities of cytokines such as IFN-γ and express activator receptors. These processes result in a cytotoxic phenotype and recruitment to primary tumor sites, with enhancement of the antitumor effect in crosstalk with CD4 and CD8 T cells and the contribution to primary tumor shrinkage (Fig. 3). In addition, CD16 cells express Fcγ RIII, which binds to the Fc region of monoclonal antibodies that bind to cancer cells and secrete perforin and granzyme B, inducing the apoptotic death of breast cancer cells and thereby exerting antibody-dependent cellular cytotoxicity (ADCC). Importantly, CD56 positivity includes CD56bright CD16- and CD56dim CD16- cells, which develop into CD56bright CD16+ and CD56dim CD16+ cells, respectively. These NK cell subsets exert potent cytotoxic activities against various cancer cells [29]. Conversely, tumor-associated CD56+ CD16- NK cells are also involved in tumor growth and angiogenesis via the production of immunosuppressive cytokines such as VEGF and TGF-β1 [17, 30], which converts CD56+ CD16+ and CD56- CD16+ cells into CD56+ CD16- and CD56- CD16- cells, respectively, thereby allowing cancer cells to escape NK-mediated antitumor immunity [30], depending on the tumor microenvironment.
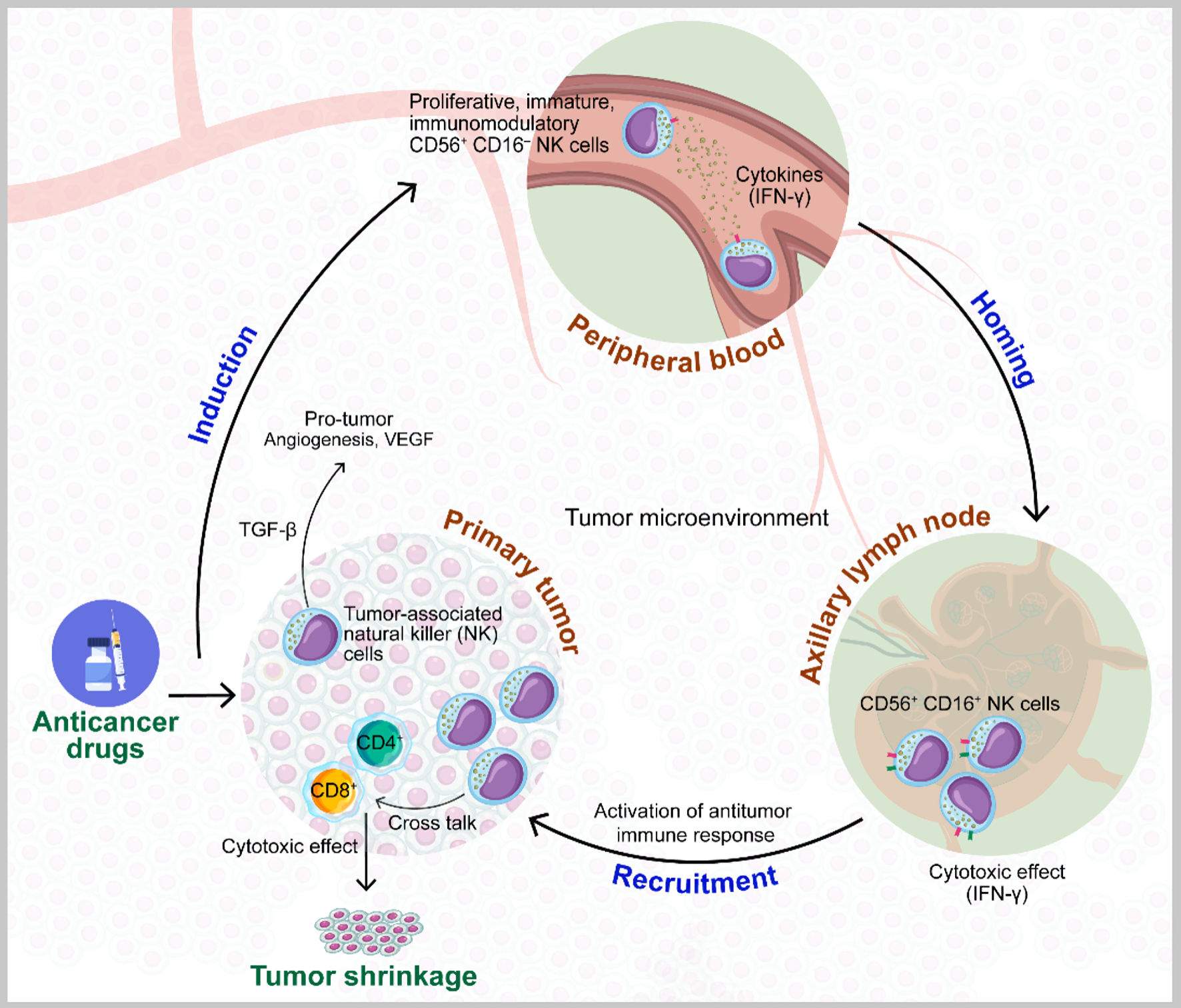 Click for large image | Figure 3. Model of the role of natural killer (NK) cells in the improvement of the effects of preoperative chemotherapy for breast cancer. Larger quantities of CD56+ CD16- NK cells in peripheral blood before and after chemotherapy are recruited to primary tumor sites, where they are converted from this immunomodulatory state with poor cytolytic effects to CD56+ CD16+ cells with cytotoxic effects via the production of interferon gamma (IFN-γ), which can contribute to primary tumor shrinkage in crosstalk with CD4 and CD8 T cells. Conversely, the phenotype of tumor-associated CD56+ CD16- NK cells is also involved in tumor growth and angiogenesis, derived from transforming growth factor beta (TGF-β) in the tumor microenvironment (TME). VEGF: vascular endothelial growth factor. |
In this study, patients with HER-2+ breast cancer treated with an anthracycline and a taxane plus Tz and/or Pz showed G ≥ 2 treatment responses. The majority of patients with L-type breast cancer also showed such treatment responses. These findings suggest that the ADCC-mediated activation of CD16+ NK cells by HER-2-targeted therapy enhances therapeutic efficacy against HER-2+ breast cancer, but that the development of CD56+ CD16+ NK cells from CD56+ CD16- NK cells enhances NK-mediated direct lysis by modulating activator receptors, also contributing to the enhancement of the therapeutic effect on primary breast tumors after preoperative chemotherapy.
The distribution of NK cell subsets varies by cancer type, suggesting that these cells are characterized by plasticity and heterogeneity in patients with cancer [12]. In patients with breast cancer, the proportion of immature, non-cytotoxic NK cell subsets increase with disease progression [14]. In this study, the median distribution of CD56- CD16+ NK cell percentages was associated significantly with TN breast cancer. CD56- CD16- NK cell subset enrichment was observed and reflected by the low cytotoxic activity of NK cells, resulting in the evasion of antitumor immunity and the promotion of tumor growth [14]. As the distribution of pNK cell subsets correlates with the distribution of NK cell subsets at tumor sites, this situation is associated with primary tumor enrichment with CD56- CD16- pNK cells [14].
In our previous studies of antitumor immune activation after preoperative chemotherapy for breast cancer, increased pNK activity, NK cell expression, and pre-chemotherapy CTLA-4 expression and decreased platelet/lymphocyte ratios and VEGF expression were associated with Ax+ disappearance, regardless of tumor subtype [8, 9, 11]. In addition, increased pre-chemotherapy CTLA-4 expression and NK, CD4, and CD8 expression at primary tumor sites were associated with G ≥ 2 therapeutic effects [8, 9, 11]. Interestingly, increased expression of the protumor factors TGF-β and interleukin 6, instead of antitumor factors, was observed in this context [8, 11]. These findings are consistent with an antitumor immune response derived from anticancer agent-induced local and systemic immune activation, and the high pre- and post-chemotherapy levels of CD56+ CD16- pNK cell subsets observed in the present study may be partly responsible for such antitumor immune activation and thus improved therapeutic effects at primary tumor sites. Importantly, small increases in CD56+ CD16+ pNK cell subsets converted from CD56+ CD16- pNK cells may play a role in tumor microenvironment regulation and facilitate therapeutic responses to anticancer drugs. Although previous studies have revealed differences in pNK subsets in benign tumors and breast cancer, including advanced stages [16], changes in specific pNK subsets after preoperative chemotherapy in patients with breast cancer have not, to our knowledge, been reported outside of this study.
Preoperative chemotherapy is generally given to patients with stages-II and -III breast cancer, resulting in downstaging for the performance of breast-conserving surgery, and pCR predicts a better prognosis than non-CR in patients with HER-2+ and TN breast cancer. Five patients in this study had de-novo stage-IV (metastatic) disease. Clinical and molecular analyses have shown that metastatic disease often shares molecular similarities with primary tumors [31], and four of these patients had G ≥ 2 therapeutic responses, which can be explained by the activation of NK-mediated antitumor immunity.
Limitations of this study include the small sample size and the lack of analysis of activator and inhibitor receptors on NK cells and NK cell subsets at tumor sites. In addition, we did not measure the pre-chemotherapy levels of cytokines such as IFN-γ and TGF-β in peripheral blood. In a preliminary study, however, we observed that IFN-γ levels before and after chemotherapy differed between some responders and non-responders, and that TGF-β levels before chemotherapy differed among non-responders. To elucidate the functional role of CD56+ CD16- pNK cells in chemotherapy effects in patients with breast cancer, we will continue to study relationships between these differences and treatment responses.
Conclusions
In this study, high pre- and post-preoperative chemotherapy levels of CD56+ CD16- NK cells may have contributed to primary breast tumor shrinkage, possibly via homing to ALNs and conversion to CD56+ CD16+ NK cells, which acquire cytotoxic activity through IFN-γ secretion and recruitment. This antitumor effect contrasts with the protumor effects of NK cells in the tumor microenvironment. The functional roles of different pNK subsets in breast cancer and the relevance of NK cells in the tumor microenvironment need to be elucidated, which may guide the design of novel therapeutic interventions targeting NK cells. Further studies are needed to more precisely determine how NK cell activation is involved in therapeutic efficacy against breast cancer, and how tumor-associated NK cells are reactivated as an antitumor effect by preoperative chemotherapy and other treatment strategies.
Acknowledgments
The authors thank SRL, Inc. (Tokyo, Japan) for the measurement of pNK cell activity and the percentages of CD4 and CD8 T cells and NK cell subtypes by two-color flow cytometry. The authors also thank the patients and their families who participated in this study.
Financial Disclosure
No specific grants were received for this study from any granting agency in the public, for-profit, or non-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
All treatments, including the publication of data obtained in this study, were performed with the informed consent of the patients.
Author Contributions
R. Kim contributed to conceptualization, formal analysis, research, materials, writing (drafting), and writing (peer review and editing); A. Kawai contributed to software, formal analysis, research, and data curation; M. Wakisaka contributed to data curation and research; M. Shimoyama contributed to data curation; N. Yasuda contributed to data curation; M. Ito was involved in data curation; T. Kin contributed to data curation and research; K. Arihiro contributed to data curation. All authors agreed to the final version and consented to publication.
Data Availability
The datasets generated and analyzed in this study are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Opzoomer JW, Sosnowska D, Anstee JE, Spicer JF, Arnold JN. Cytotoxic chemotherapy as an immune stimulus: a molecular perspective on turning up the immunological heat on cancer. Front Immunol. 2019;10:1654.
doi pubmed pmc - Salgado R, Denkert C, Campbell C, Savas P, Nuciforo P, Aura C, de Azambuja E, et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO trial. JAMA Oncol. 2015;1(4):448-454.
doi pubmed pmc - Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):179.
doi pubmed pmc - He L, Wang Y, Wu Q, Song Y, Ma X, Zhang B, Wang H, et al. Association between levels of tumor-infiltrating lymphocytes in different subtypes of primary breast tumors and prognostic outcomes: a meta-analysis. BMC Womens Health. 2020;20(1):194.
doi pubmed pmc - Denkert C, von Minckwitz G, Darb-Esfahani S, Lederer B, Heppner BI, Weber KE, Budczies J, et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 2018;19(1):40-50.
doi pubmed - Goker E, Hendriks MP, van Tilburg M, Barcaru A, Mittempergher L, van Egmond A, Kleijn M, et al. Treatment response and 5-year distant metastasis-free survival outcome in breast cancer patients after the use of MammaPrint and BluePrint to guide preoperative systemic treatment decisions. Eur J Cancer. 2022;167:92-102.
doi pubmed - Muraro E, Comaro E, Talamini R, Turchet E, Miolo G, Scalone S, Militello L, et al. Improved Natural Killer cell activity and retained anti-tumor CD8(+) T cell responses contribute to the induction of a pathological complete response in HER2-positive breast cancer patients undergoing neoadjuvant chemotherapy. J Transl Med. 2015;13:204.
doi pubmed pmc - Kim R, Kawai A, Wakisaka M, Funaoka Y, Yasuda N, Hidaka M, Morita Y, et al. A potential role for peripheral natural killer cell activity induced by preoperative chemotherapy in breast cancer patients. Cancer Immunol Immunother. 2019;68(4):577-585.
doi pubmed pmc - Kim R, Kawai A, Wakisaka M, Sawada S, Shimoyama M, Yasuda N, Hidaka M, et al. Immune factors associated with the pathological and therapeutic effects of preoperative chemotherapy in patients with breast cancer. Transl Oncol. 2021;14(1):100927.
doi pubmed pmc - Zhang J, Pan S, Jian C, Hao L, Dong J, Sun Q, Jin H, et al. Immunostimulatory properties of chemotherapy in breast cancer: from immunogenic modulation mechanisms to clinical practice. Front Immunol. 2021;12:819405.
doi pubmed pmc - Kim R, Kawai A, Wakisaka M, Sawada S, Shimoyama M, Yasuda N, Hidaka M, et al. Immune correlates of the differing pathological and therapeutic effects of neoadjuvant chemotherapy in breast cancer. Eur J Surg Oncol. 2020;46(1):77-84.
doi pubmed - Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol Cancer. 2020;19(1):120.
doi pubmed pmc - Cooper MA, Fehniger TA, Caligiuri MA. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22(11):633-640.
doi pubmed - Moretta L, Bottino C, Pende D, Mingari MC, Biassoni R, Moretta A. Human natural killer cells: their origin, receptors and function. Eur J Immunol. 2002;32(5):1205-1211.
doi pubmed - Abdel-Latif M, Youness RA. Why natural killer cells in triple negative breast cancer? World J Clin Oncol. 2020;11(7):464-476.
doi pubmed pmc - Mamessier E, Pradel LC, Thibult ML, Drevet C, Zouine A, Jacquemier J, Houvenaeghel G, et al. Peripheral blood NK cells from breast cancer patients are tumor-induced composite subsets. J Immunol. 2013;190(5):2424-2436.
doi pubmed - Levi I, Amsalem H, Nissan A, Darash-Yahana M, Peretz T, Mandelboim O, Rachmilewitz J. Characterization of tumor infiltrating natural killer cell subset. Oncotarget. 2015;6(15):13835-13843.
doi pubmed pmc - Wang L, Chen Z, Liu G, Pan Y. Functional crosstalk and regulation of natural killer cells in tumor microenvironment: Significance and potential therapeutic strategies. Genes Dis. 2023;10(3):990-1004.
doi pubmed pmc - Chan A, Hong DL, Atzberger A, Kollnberger S, Filer AD, Buckley CD, McMichael A, et al. CD56bright human NK cells differentiate into CD56dim cells: role of contact with peripheral fibroblasts. J Immunol. 2007;179(1):89-94.
doi pubmed - Hanna J, Bechtel P, Zhai Y, Youssef F, McLachlan K, Mandelboim O. Novel insights on human NK cells' immunological modalities revealed by gene expression profiling. J Immunol. 2004;173(11):6547-6563.
doi pubmed - Beziat V, Duffy D, Quoc SN, Le Garff-Tavernier M, Decocq J, Combadiere B, Debre P, et al. CD56brightCD16+ NK cells: a functional intermediate stage of NK cell differentiation. J Immunol. 2011;186(12):6753-6761.
doi pubmed - Hu Z, Xu X, Wei H. The adverse impact of tumor microenvironment on NK-cell. Front Immunol. 2021;12:633361.
doi pubmed pmc - Allan DS, Rybalov B, Awong G, Zuniga-Pflucker JC, Kopcow HD, Carlyle JR, Strominger JL. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40(8):2289-2295.
doi pubmed pmc - Brierley JD, Gospodarowicz MK, Wittekind C(eds). TNM Classification of Malignant Tumours. 8th ed. New Jersey: WILEY-Blackwell; 2017.
- Kurosumi M, Akashi-Tanaka S, Akiyama F, Komoike Y, Mukai H, Nakamura S, Tsuda H, et al. Histopathological criteria for assessment of therapeutic response in breast cancer (2007 version). Breast Cancer. 2008;15(1):5-7.
doi pubmed - Horii R, Akiyama F. Histological assessment of therapeutic response in breast cancer. Breast Cancer. 2016;23(4):540-545.
doi pubmed - Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, Bonnefoi H, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164-172.
doi pubmed - Zhang GC, Zhang YF, Xu FP, Qian XK, Guo ZB, Ren CY, Yao M. Axillary lymph node status, adjusted for pathologic complete response in breast and axilla after neoadjuvant chemotherapy, predicts differential disease-free survival in breast cancer. Curr Oncol. 2013;20(3):e180-192.
doi pubmed pmc - Takahashi E, Kuranaga N, Satoh K, Habu Y, Shinomiya N, Asano T, Seki S, et al. Induction of CD16+ CD56bright NK cells with antitumour cytotoxicity not only from CD16- CD56bright NK Cells but also from CD16- CD56dim NK cells. Scand J Immunol. 2007;65(2):126-138.
doi pubmed - Mamessier E, Sylvain A, Thibult ML, Houvenaeghel G, Jacquemier J, Castellano R, Goncalves A, et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J Clin Invest. 2011;121(9):3609-3622.
doi pubmed pmc - Pusztai L, Rozenblit M, Dubsky P, Bachelot T, Kirby AM, Linderholm BK, White JR, et al. De novo oligometastatic breast cancer. J Clin Oncol. 2023;41(34):5237-5241.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


