| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 4, August 2024, pages 579-591
Impact of Lymph Node Dissection for Patients With Clinically Node-Negative Intrahepatic Cholangiocarcinoma: A Multicenter Cohort Study
Meng Shaa, e, f, Jie Caoa, e, Cheng Lin Qinb, e, Jian Zhangc, e, Chao Fand, Zhe Lia, Ying Tonga, Lei Xiaa, Jian Jun Zhanga, Qiang Xiaa, f
aDepartment of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
bDepartment of Hepatobiliary Surgery, The First People’s Hospital of Yancheng, Jiangsu, China
cDepartment of Hepatobiliary Surgery, The Second Affiliated Hospital of Xi’an Jiaotong University, Shanxi, China
dDepartment of Mathematics, The University of California San Diego, La Jolla, CA, USA
eThese authors contributed equally to this work.
fCorresponding Author: Meng Sha, Department of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China; Qiang Xia, Department of Liver Surgery, Renji Hospital, School of Medicine, Shanghai Jiao Tong University, Shanghai 200127, China
Manuscript submitted May 16, 2024, accepted June 14, 2024, published online July 5, 2024
Short title: LND for Clinically Node-Negative ICC
doi: https://doi.org/10.14740/wjon1895
| Abstract | ▴Top |
Background: Lymph node status is a prominent prognostic factor for intrahepatic cholangiocarcinoma (ICC). However, the prognostic value of performing lymph node dissection (LND) in patients with clinical node-negative ICC remains controversial. The aim of this study was to evaluate the clinical value of LND on long-term outcomes in this subgroup of patients.
Methods: We retrospectively analyzed patients who underwent radical liver resection for clinically node-negative ICC from three tertiary hepatobiliary centers. The propensity score matching analysis at 1:1 ratio based on clinicopathological data was conducted between patients with and without LND. Recurrence-free survival (RFS) and overall survival (OS) were compared in the matched cohort.
Results: Among 303 patients who underwent radical liver resection for ICC, 48 patients with clinically positive nodes were excluded, and a total of 159 clinically node-negative ICC patients were finally eligible for the study, with 102 in the LND group and 57 in the non-LND group. After propensity score matching, two well-balanced groups of 51 patients each were analyzed. No significant difference of median RFS (12.0 vs. 10.0 months, P = 0.37) and median OS (22.0 vs. 26.0 months, P = 0.47) was observed between the LND and non-LND group. Also, LND was not identified as one of the independent risks for survival. Among 51 patients who received LND, 11 patients were with positive lymph nodes (lymph node metastasis (LNM) (+)) and presented significantly worse outcomes than those with LND (-). On the other hand, postoperative adjuvant therapy was the independent risk factor for both RFS (hazard ratio (HR): 0.623, 95% confidence interval (CI): 0.393 - 0.987, P = 0.044) and OS (HR: 0.585, 95% CI: 0.359 - 0.952, P = 0.031). Furthermore, postoperative adjuvant therapy was associated with prolonged survivals of non-LND patients (P = 0.02 for RFS and P = 0.03 for OS).
Conclusions: Based on the data, we found that LND did not significantly improve the prognosis of patients with clinically node-negative ICC. Postoperative adjuvant therapy was associated with prolonged survival of ICC patients, especially in non-LND individuals.
Keywords: Intrahepatic cholangiocarcinoma; Liver resection; Lymph node dissection; Lymph node metastasis
| Introduction | ▴Top |
Intrahepatic cholangiocarcinoma (ICC), the second most common primary liver malignancy, has increased dramatically across the world in the past decades [1]. Radical surgery remains the potential curative treatment that improves survival outcomes [2, 3]. However, the 5-year overall survival (OS) rate of patients was barely at 30% even after curative liver resection [4, 5]. For locally advanced or metastatic ICC, the latest guidelines have recommended the use of gemcitabine- or capecitabine-based chemotherapy to provide survival advantage [6], however, the median OS was still far from satisfactory. Recently, several clinical trials including targeted therapies and immune checkpoint inhibitors, are currently evaluating the role of novel therapeutic approaches in patients with advanced ICC [7-9]. The studies are still ongoing, and the results are yet to be conclusively confirmed whether to provide survival benefits.
Among all the risk factors, lymph node status is acknowledged as one of the most prominent prognostic factors associated with tumor recurrence and poor outcomes [10]. Therefore, TNM staging system recommends the dissection of lymph nodes for accurate staging and better outcomes [11]. In clinical practice, standard lymph node dissection (LND) has been widely accepted in patients with preoperative evidence of lymph node metastasis (LNM) (clinically node-positive) [12]. However, it remains controversial to perform LND in patients without preoperative evidence of LNM (clinically node-negative) [13]. The main reason is the lack of evidence of its impact on survival benefits [14]. Besides, additional LND for clinically node-negative patients may lead to hepatic injury or chylous leak that increases risk of postoperative complications [15].
Since most retrospective studies focused on prognostic value of LND in ICC patients regardless of preoperative lymph node status, the goal of our study is to evaluate the long-term outcomes of LND for patients with clinically node-negative ICC. In addition, we also aimed to identify which subgroup of patients may benefit most from the surgery.
| Materials and Methods | ▴Top |
Patient selection
Patients who underwent radical hepatectomy for ICC between January 2007 and December 2019 were retrospectively evaluated from three tertiary referral centers in China (Renji Hospital, Shanghai; the Second Affiliated Hospital of Xi’an Jiaotong University, Shanxi; the First People’s Hospital of Yancheng, Jiangsu). Patients were enrolled in the study meeting the inclusion criteria: 1) Pathological confirmation of ICC; 2) No LNM or extrahepatic metastasis identified by preoperative imaging tests; 3) Hepatectomy performed with R0 resection. Patients who received preoperative adjuvant therapy were excluded from the study. In addition, postoperative death within 1 month was also excluded (Fig. 1). The Institutional Review Board has approved and confirmed the study. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
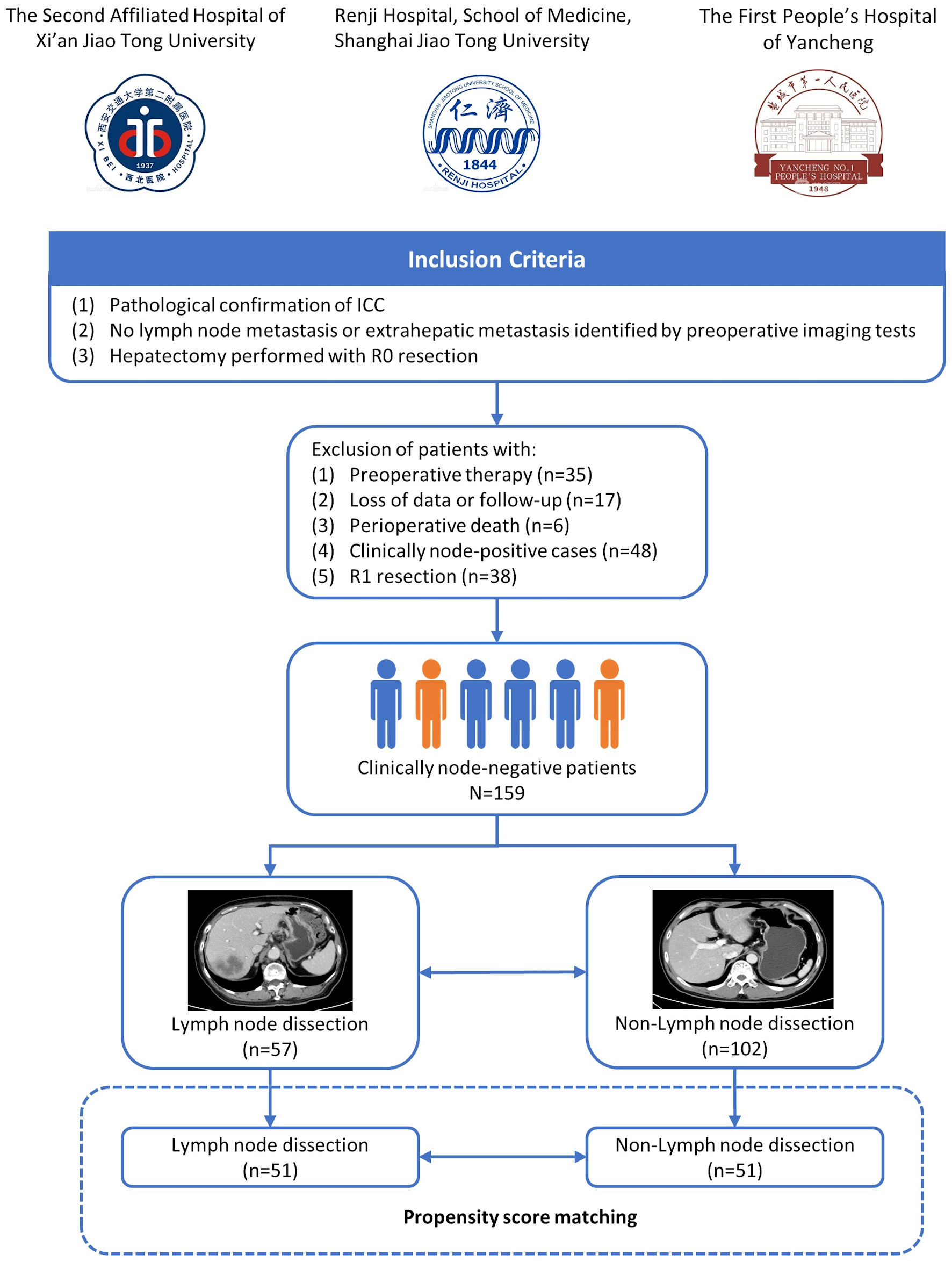 Click for large image | Figure 1. Flowchart of patients’ enrollment. ICC: intrahepatic cholangiocarcinoma. |
Definition of clinically node-negative ICC
Clinically node-negative ICC was defined as patients with no suspicious or positive LNM evaluated by preoperative radiology findings [16]. Presence of lymph nodes > 1 cm, contrast enhancement at computed tomography (CT) or magnetic resonance imaging (MRI) and central necrosis or extra-nodal extension was proposed as clinically node-positive ICC [17, 18]. Patients without LND were defined as non-LND, while patients receiving LND were divided into LNM (+) and LNM (-) according to postoperative pathological status of lymph nodes (Fig. 2).
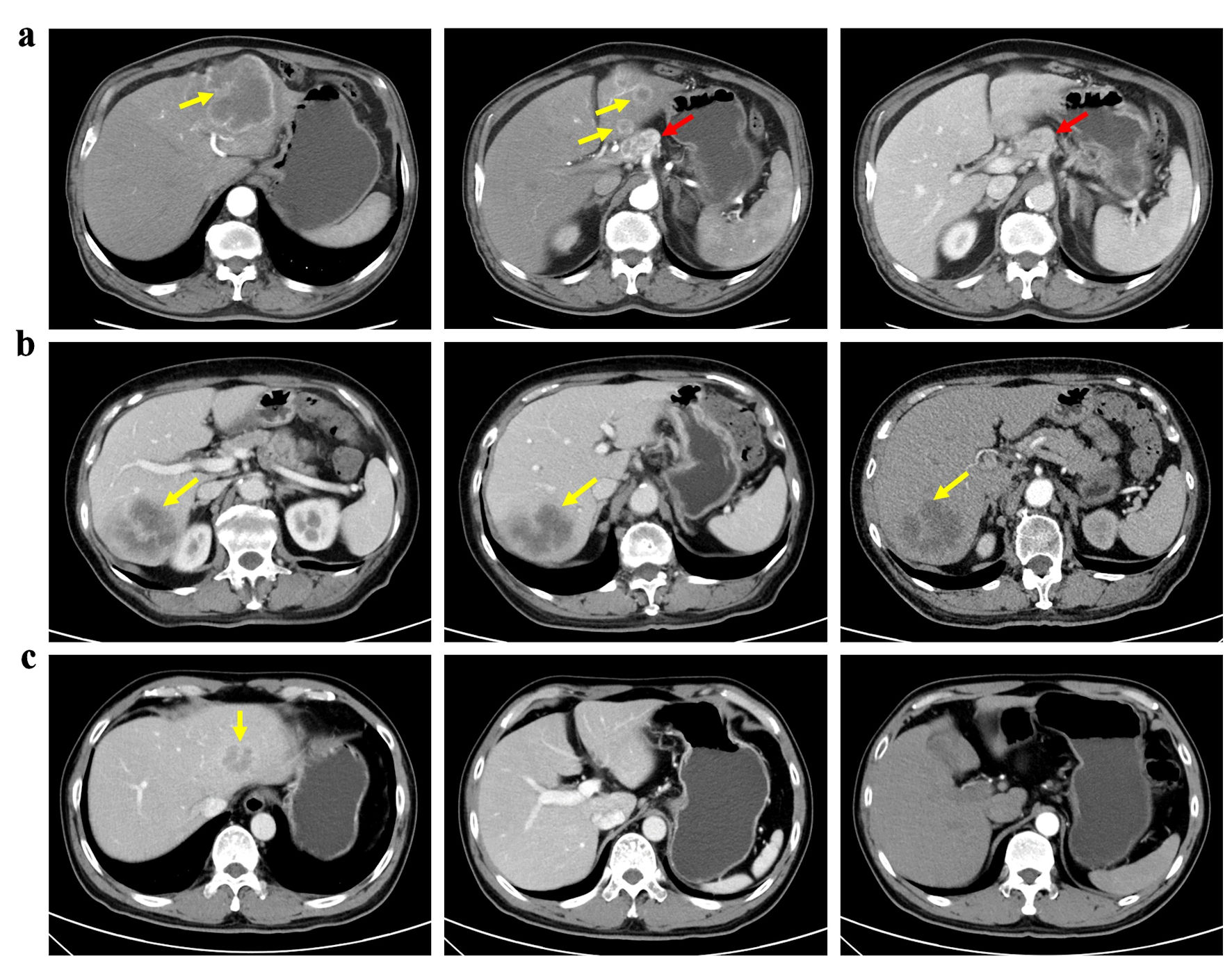 Click for large image | Figure 2. Patients with different lymph node status of ICC. (a) A patient with clinically node-positive ICC received left hemi-hepatectomy and lymph node dissection. (b) A patient with clinically node-negative ICC underwent both liver resection and lymph node dissection based on suspicion of lymph node metastasis at multidisciplinary team discussion. Postoperative pathology confirmed no lymph node metastasis. (c) A patient with clinically node-negative ICC underwent liver resection only. Yellow arrows: tumor; red arrows: lymph node. |
Operation and treatment strategy
The operative strategies were determined by senior surgeons on the basis of patients’ clinical conditions including size, number and location of tumor, vascular involvement, liver function reserve and volume of residual liver. LND was performed when: 1) Patients were suspicious of LNM at a weekly multidisciplinary team discussion before operation; or 2) Enlarged lymph nodes were detected during surgical findings. The extent of LND in the present study included: 1) Lymph nodes located around the hepatoduodenal ligament and the common hepatic artery (station 12 and 8); 2) Retro-pancreatic lymph nodes (station 13, for right-sided tumors); and 3) Lymph nodes around the cardiac portion of the stomach and along the lesser curvature (station 7, for left-sided tumors) (Fig. 3).
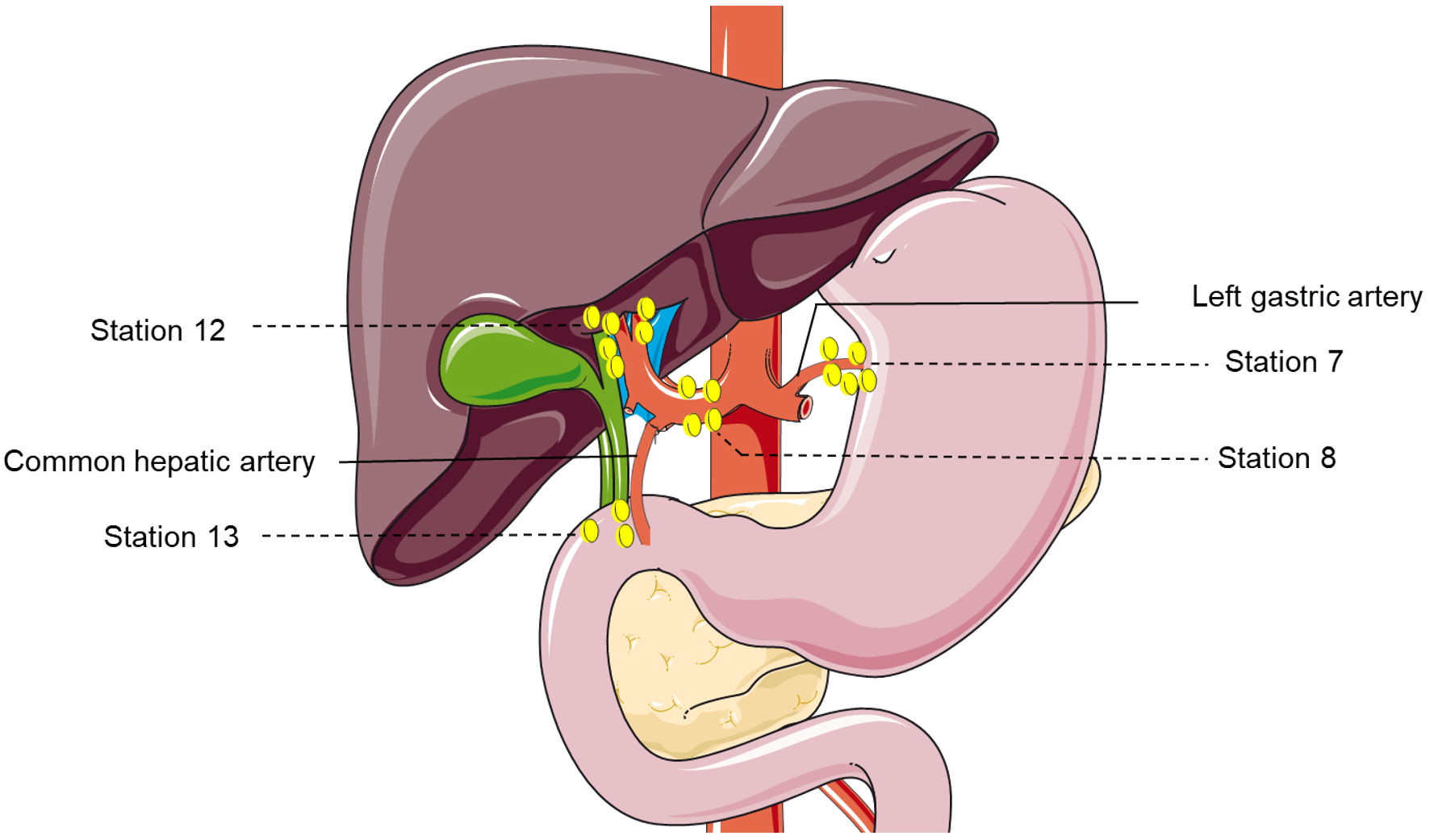 Click for large image | Figure 3. The lymph node dissection map of patients with suspected positive lymph nodes. Including: 1) Lymph nodes located around the hepatoduodenal ligament and the common hepatic artery (stations 12 and 8); 2) Retro-pancreatic lymph nodes (station 13, for right-sided tumors); and 3) Lymph nodes around the cardiac portion of the stomach and along the lesser curvature (station 7, for left-sided tumors). |
Postoperative adjuvant therapy was determined by multidisciplinary team discussion on a case-by-case basis. Generally, the regimen included transarterial chemoembolization, four to six courses of cisplatin or gemcitabine-based chemotherapy, radiotherapy or chemoradiotherapy.
Data collection and follow-up
Demographic data were collected including gender, age, hepatitis virus infection, Karnofsky Performance Status (KPS) score, cirrhosis and carbohydrate antigen 19-9 (CA 19-9) levels. Tumor characteristics through radiological and pathological findings included tumor size, tumor number, vascular invasion, tumor grade, perineural invasion, number of harvested lymph nodes and resection margin. In addition, intra- and postoperative parameters of surgical time, blood loss, hospital stays, and major complications were also collected. The Dindo-Clavien classification was used to evaluate postoperative complications which were considered as “major” when graded > II [19].
All patients were followed-up once per 3 months within 2 years after surgery and once per 6 months thereafter. The clinical monitoring consisted of physical assessment, liver function tests, CA 19-9 level, as well as abdominal ultrasound. CT or MRI scans of the chest and abdomen were performed once every 6 months to detect early recurrence. The positron emission tomography-computed tomography (PET-CT) was conducted when tumor recurrence was highly suspected. OS was calculated as the interval between the date of surgery and the date of death, while recurrence-free survival (RFS) was calculated as interval between surgery and presence of tumor recurrence at any site.
Statistical analysis
Propensity score matching (PSM) was conducted to minimize the bias between LND group and non-LND group [20]. Variables including gender, age, hepatitis virus infection, KPS score, cirrhosis, CA 19-9 level, tumor diameter, tumor number, vascular invasion, tumor grade and perineural invasion underwent 1:1 nearest-available matching of the propensity score with a caliper width of 0.20. Categorical variables are presented as numbers with percentage, and continuous variables are expressed as median with interquartile range. The correlations between LND and clinicopathological characteristics were estimated with χ2 or Fisher exact test before and after PSM. Continuous variables of intra- and postoperative outcomes were compared using Student’s t-test or Mann-Whitney U test. Cox hazards proportional regression was performed to identify independent risk factors associated with RFS and OS. Kaplan-Meier analysis and log-rank test were used to estimate and compare survival curves. All analyses were performed using SPSS (version 24.0) with P value < 0.05 determined as statistical significance.
| Results | ▴Top |
Initially, 303 patients who underwent curative-intent resection for ICC between January 2007 and December 2019 were screened for the study. Fifty-eight patients were excluded due to preoperative therapy (n = 35), loss of data or follow-up (n = 17) and perioperative death (n = 6) based on the study criteria. Of the remaining patients, 48 patients with clinically positive nodes and 38 patients who underwent R1 resection did not meet the selection criteria. Finally, a total of 159 patients with clinically node-negative ICC were enrolled in the present study.
Patient clinicopathological characteristics
Of the 159 patients included in the study, 57 underwent LND and 102 did not. The baseline clinicopathological characteristics of patients were summarized in Table 1. Before PSM, presence of cirrhosis was significantly higher in the LND group than that in the non-LND group (P < 0.001). After PSM, 11 selected variables (gender, age, hepatitis B virus (HBV) infection, cirrhosis, KPS score, CA 19-9 level, tumor diameter, number, differentiation, vascular invasion and perineural invasion) showed no significant difference between the two groups (all P > 0.05).
 Click to view | Table 1. Correlation Between Lymph Node Dissection and Clinicopathological Characteristics in Patients Matched by LND Before and After PSM |
Intra- and postoperative outcomes
The median retrieval of lymph nodes was 4 in the LND group (range 1 - 21). As shown in Table 2, surgical time, blood loss and incidence of major complications related to surgery did not differ between LND group and non-LND group before and after PSM (all P > 0.05). Nearly half of patients received postoperative adjuvant therapy both in the LND and non-LND group. No significant difference was observed in the proportion of postoperative treatment before and after PSM (P > 0.05). It is noted that hospital stay was longer in the LND group than that in the non-LND group before PSM (10 days vs. 9 days, P = 0.021). The difference still exists after PSM (10 days vs. 9 days, P = 0.049).
 Click to view | Table 2. Intra- and Postoperative Outcomes in Patients Matched by LND Before and After PSM |
Survival outcomes
In the crude cohort, median RFS was similar between the LND group and non-LND group (12.0 vs. 10.0 months, P = 0.37) (Fig. 4a). For median OS, it was slightly shorter in the LND group than that in the non-LND group with no significant difference (22.0 vs. 26.0 months, P = 0.47) (Fig. 4b). The 1-, 3- and 5-year survival rates in the two groups were 82.4% vs. 60.8%, 33.9% vs. 43.0% and 26% vs. 38.6%, respectively.
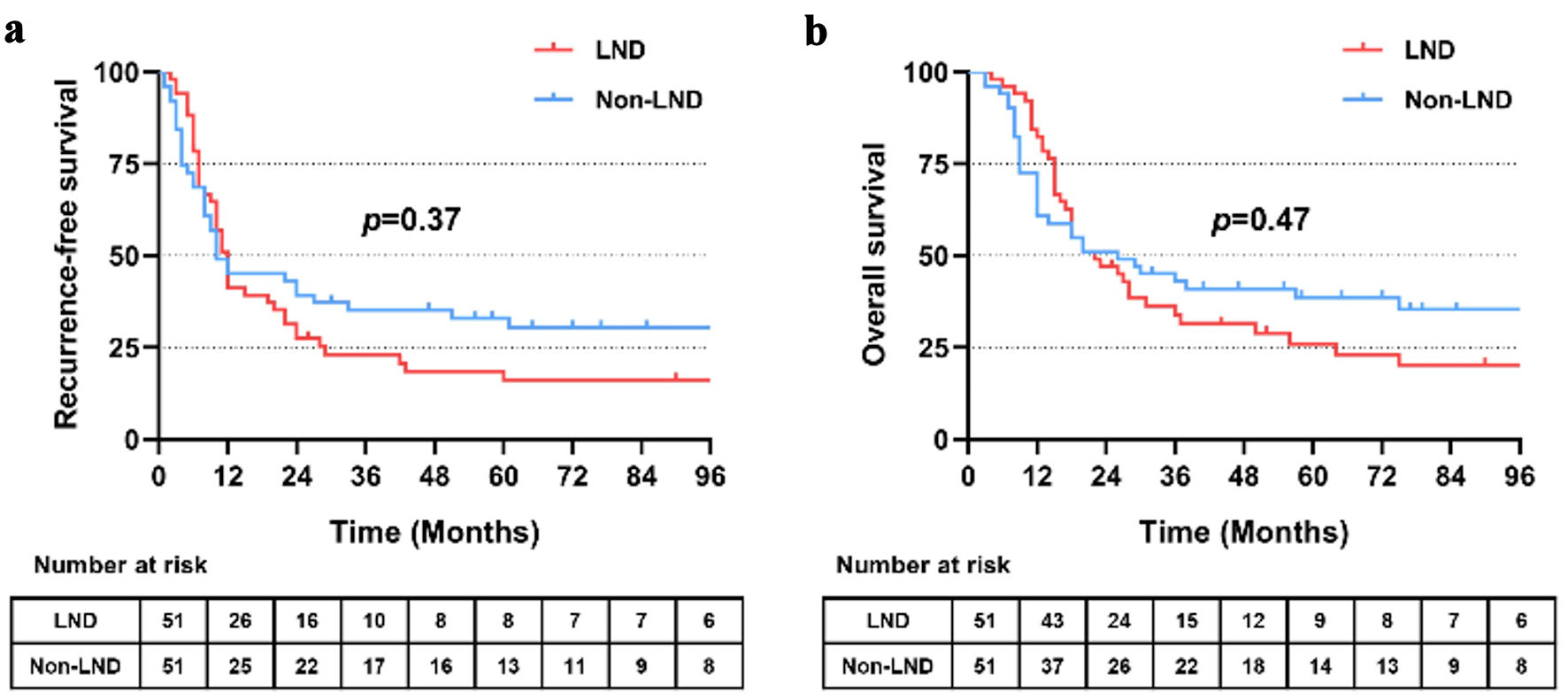 Click for large image | Figure 4. Recurrence-free (a) and overall (b) survival of patients receiving lymph node dissection or not after propensity score matching (PSM). LND: lymph node dissection. |
Among 51 patients who received LND, 11 patients presented with positive lymph nodes (LNM (+)) and 40 patients presented with LNM (-) confirmed by pathological findings. The presence of LNM significantly affected RFS and OS. Patients with LNM (-) showed significantly better outcomes than those with LNM (+), with a median RFS of 17.0 vs. 10.0 months (P = 0.03) and a median OS of 27.0 vs. 15.0 months (P = 0.02) (Fig. 5a, b). Of note, no significant difference of RFS or OS was observed between patients with non-LND and LNM (-) (P = 0.65 and 0.60, respectively) (Fig. 5c, d).
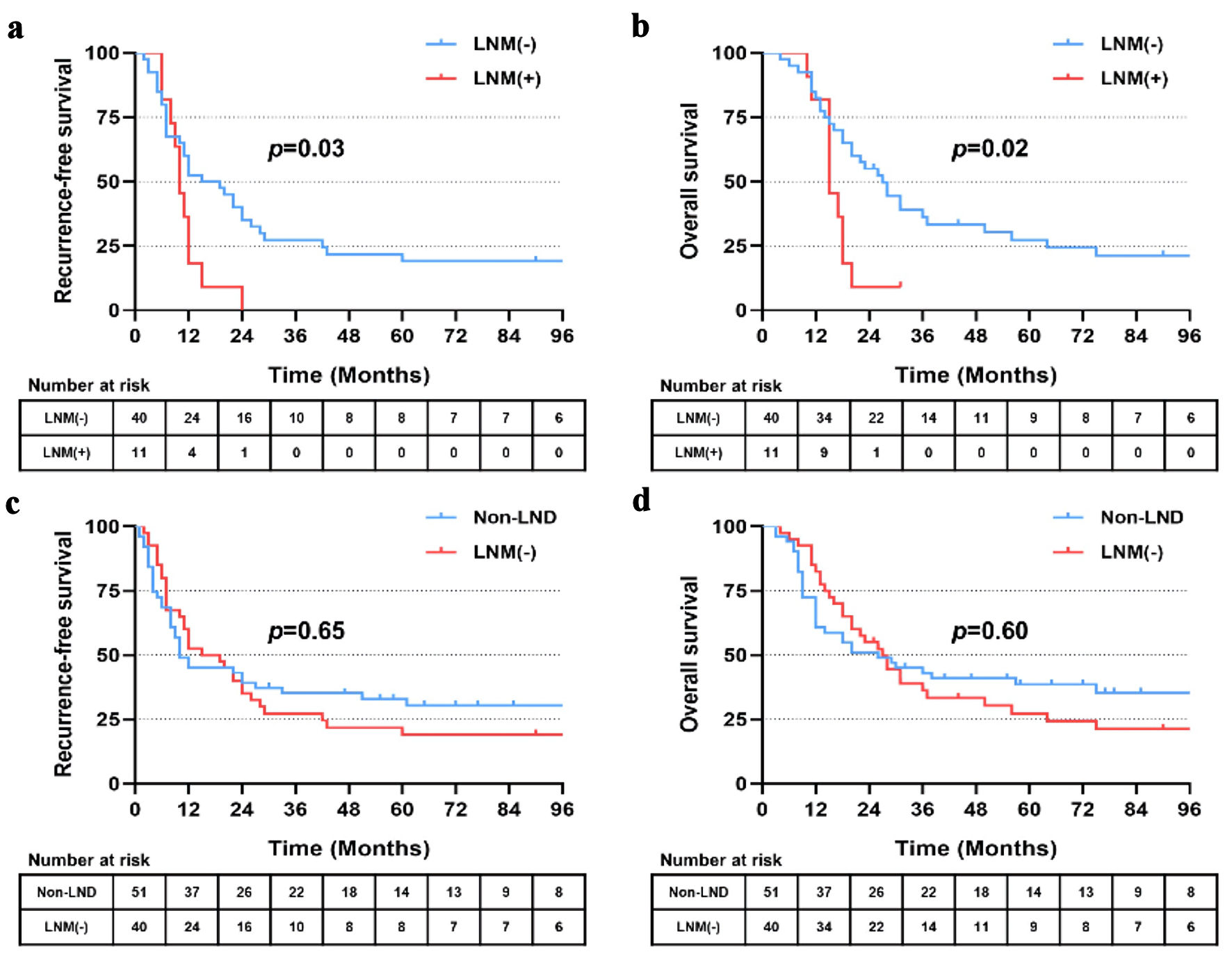 Click for large image | Figure 5. Recurrence-free (a) and overall (b) survival of patients with lymph node metastasis (LNM (+)) and no lymph node metastasis (LNM (-)). Recurrence-free (c) and overall (d) survival of patients with hepatectomy alone (non-LND) and LNM (-). LND: lymph node dissection. |
Independent prognostic factor associated with survival
In the matched cohort, tumor diameter, tumor number, vascular invasion and postoperative adjuvant therapy were identified to be related with RFS through univariate analysis (Table 3). These factors were further evaluated using multivariate analysis. It turns out that only tumor diameter (hazard ratio (HR): 2.027, 95% confidence interval (CI): 1.231 - 3.338, P = 0.005) and postoperative adjuvant therapy (HR: 0.623, 95% CI: 0.393 - 0.987, P = 0.044) were independent risk factors for RFS. For OS, tumor diameter (HR: 2.172, 95% CI: 1.304 - 3.618, P = 0.003), tumor number (HR: 2.130, 95% CI: 1.223 - 3.711, P = 0.008) and postoperative adjuvant therapy (HR: 0.585, 95% CI: 0.359 - 0.952, P = 0.031) were determined as independent risk factors through univariate and multivariate analysis (Table 4). It is noted that LND was not prognostic factors for either RFS or OS.
 Click to view | Table 3. Univariate and Multivariate Analysis of Prognosis Factors for Recurrence-Free Survival in Patients Who Underwent Resection for ICC After PSM |
 Click to view | Table 4. Univariate and Multivariate Analysis of Prognosis Factors for Overall Survival in Patients Who Underwent Resection for ICC After PSM |
Effect of postoperative treatment of ICC
In the matched cohort, 44 patients received postoperative adjuvant therapy, including 22 patients in the non-LND group, 16 patients in the LNM (-) group and six patients in the LNM (+) group. Patients who received adjuvant therapy showed significantly longer RFS (22.0 vs. 9.5 months, P = 0.04) and OS (36.0 vs. 17.0 months, P = 0.04) than those without adjuvant therapy (Figs. 6a, 7a). Further stratified analysis found that patients in the subgroup of non-LND benefited most from postoperative therapy (P = 0.02 for RFS, and P = 0.03 for OS) (Figs. 6b, 7b). However, no significant benefits of RFS or OS were observed by postoperative treatment in the subgroups of LNM (-) and LNM (+) (all P > 0.05) (Figs. 6c, d and 7c, d).
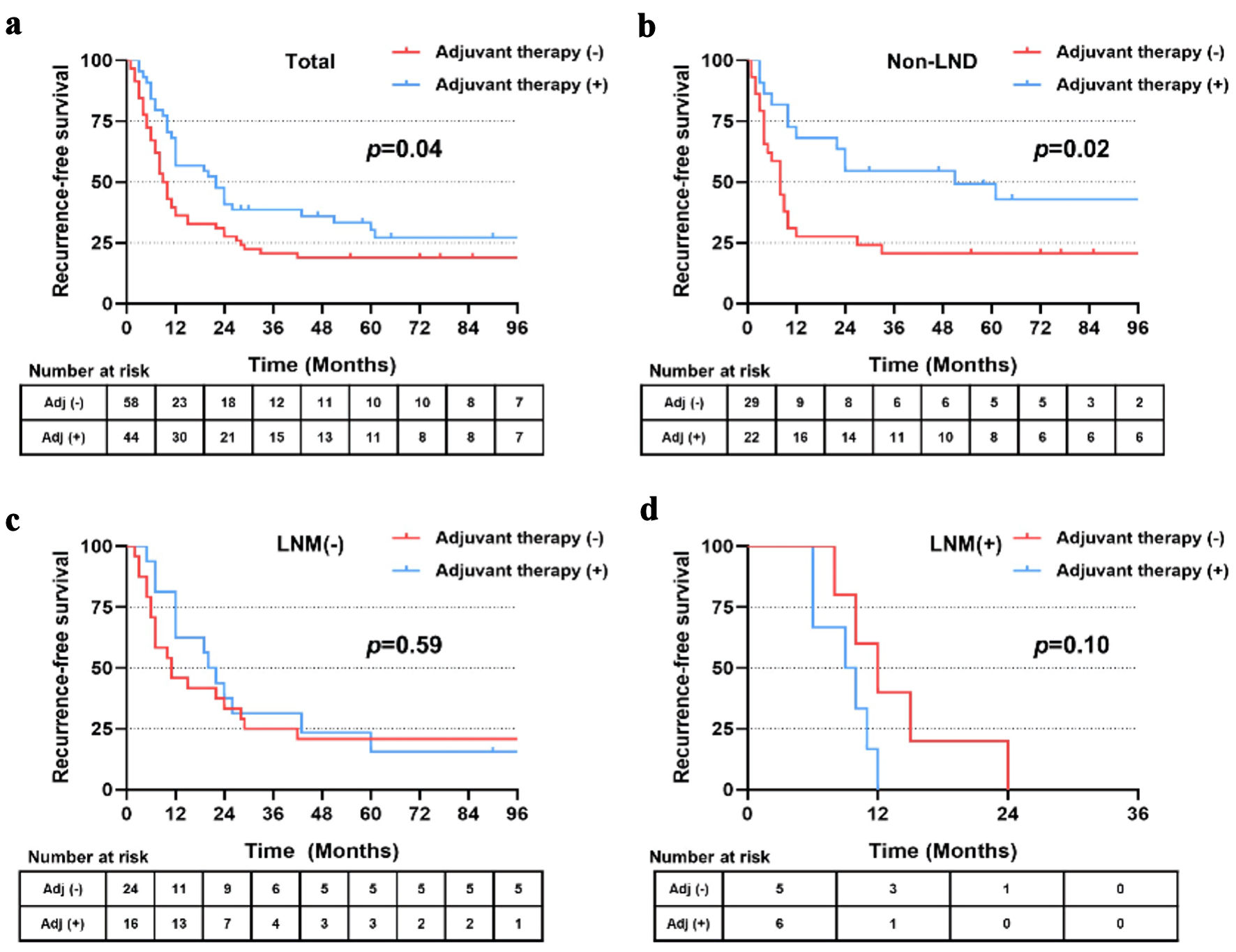 Click for large image | Figure 6. (a-d) Recurrence-free survival of patients in total, with non-LND, LNM (-) and LNM (+) receiving postoperative adjuvant therapy or not after PSM. LND: lymph node dissection; LNM: lymph node metastasis; PSM: propensity score matching. |
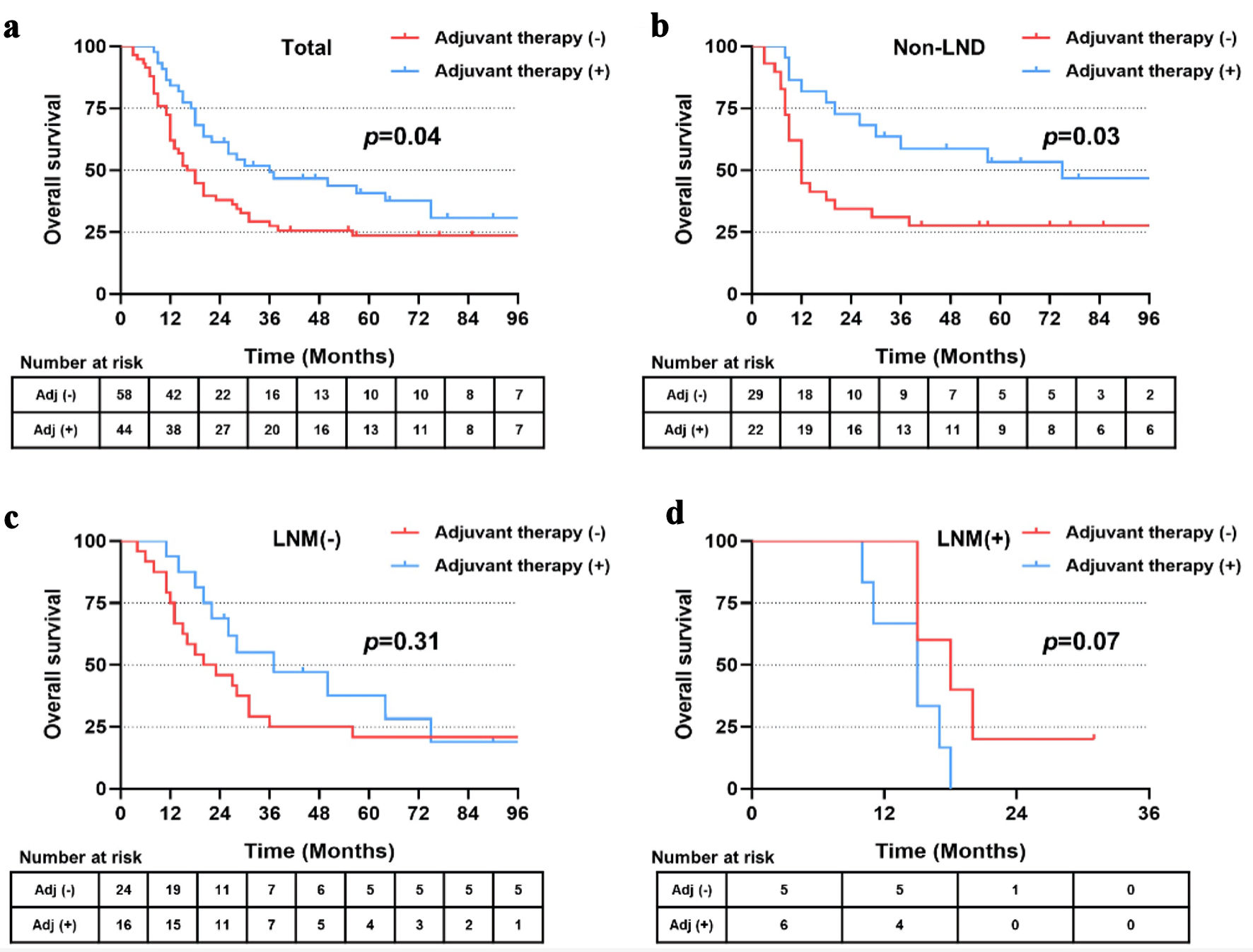 Click for large image | Figure 7. (a-d) Overall survival of patients in total, with non-LND, LNM (-) and LNM (+) receiving postoperative adjuvant therapy or not after PSM. LND: lymph node dissection; LNM: lymph node metastasis; PSM: propensity score matching. |
| Discussion | ▴Top |
Lymph node status is one of the most crucial factors for accurate staging and predicting long-term outcomes of ICC [21, 22]. However, LND has long been debated in the surgical resection of ICC, especially for clinically node-negative tumors [23]. Our present study evaluated the prognostic value of LND for patients with clinically node-negative ICC based on patients from three tertiary referral centers in China. The results revealed that LND did not bring survival benefits for patients with clinically node-negative ICC. While postoperative adjuvant therapy for non-LND patients was associated with prolonged survival of ICC patients.
Routine LND of clinically node-negative ICC has not come to consensus in the guidelines, resulting in heterogeneous practices in ICC operation [24]. In the current study, there was no significant difference of median RFS and OS between patients in LND and non-LND group, which was coincident with previous study. While, Japanese and another Chinese Fujian cohort reported survival benefits and advocated the LND for patients with ICC [25, 26]. The difference can be attributed to several reasons as follows: Firstly, in Fujian cohort, 40.6% of patients with clinically negative ICC receiving LND were confirmed to be LNM postoperatively. The high false-negative rate significantly lowered the survivals of patients in the LND group, while the rate in our cohort was only 21%. Second, at least six lymph nodes are recommended to be harvested according to the Eighth American Joint Committee on Cancer (AJCC) guidelines [27]. In the current study, the median retrieval nodes in the LND group were only 4 (range 1 - 21) due to different surgeon experiences. Inadequate LND and potential nodal metastasis may impact the survival rate. In addition, a higher proportion of postoperative adjuvant therapy (22 of 51, 43.1%) was reported in our non-LND group. More antitumor therapy may inhibit hidden tumor cells and bring survival benefits [28].
Though the present study did not show survival benefits by LND, this is not to deny the role of preoperative lymph node staging for ICC. Actually, in our study, clinically node-negative patients with postoperative LNM (+) showed significantly worse outcomes than those with LNM (-). The result confirms the importance of preoperative evaluation for lymph node status [29]. Under this setting, it highlights the accuracy of preoperative imaging evaluation. However, previous studies reported that the sensitivity and specificity of CT/MRI varied from 35.0% to 78.2% [30, 31], which strongly calls for more precise detection methods. Recent studies reported that 18-F fluorodeoxyglucose (FDG) PET-CT [32, 33] showed significantly higher sensitivity, specificity and accuracy than conventional CT/MRI in diagnosing regional LNM, which may facilitate tumor staging in ICC. Besides, artificial intelligence-based radiomics [34, 35] also performed well in metastatic nodal discrimination, enabling accurate N staging of ICC and patient risk stratification.
Postoperative adjuvant therapy is another important issue to discuss. Our study showed postoperative adjuvant therapy was the independent risk factor for both RFS and OS. Patients who received adjuvant therapy revealed significantly better outcomes than those without adjuvant therapy. It is interesting to further find that patients in the subgroup of non-LND benefited most from postoperative therapy. The results were consistent with the current Eighth National Comprehensive Cancer Network (NCCN) guidelines that suggested systemic therapy or gemcitabine-/capecitabine-based chemotherapy for patients with low-risk resected ICC [36-38]. It is reported that more than 50% of recurrences occurred in liver only even after adequate LND [27], which implies that most hidden tumor cells exist within the liver or bloodstream rather than lymph nodes [39]. This may explain why patients with non-LND get better outcomes from adjuvant therapy to eliminate residual disease. It also suggests a potential reevaluation of surgical strategies in favor of less invasive approaches complemented by adjuvant therapies. On the other hand, survival outcomes did not differ in patients of LND (+) with and without adjuvant therapy. It was supported by the recent STAMP trial [40], which revealed that adjuvant gemcitabine plus cisplatin did not improve survival outcomes in resected lymph node-positive cholangiocarcinoma. However, it is noted that the limited number of patients in the LNM (-) subgroup of our study may impede the evaluation of postoperative treatment in these patients. Additionally, detailed protocol of adjuvant therapy varied between each center, which calls for prospective clinical trials of consistent adjuvant regimen. Currently, rapid developments in systemic therapy, including immune checkpoint inhibitors and multi-kinase inhibitors, have demonstrated convincing effects for ICC [41, 42]. More promising adjuvant therapy approaches will improve the prognosis of ICC in future.
Our study has some potential limitations. Firstly, the retrospective nature of the study unavoidably introduces biases in data collection and patient selection. Although a PSM analysis was conducted to minimize the drawback, the limited number of patients may impact the statistical power of the study and the generalizability of the findings. Secondly, precise evaluation of imaging in determining lymph node status is difficult to made based on the current methods. Imaging modalities can sometimes fail to detect microscopic metastases, leading to a potential underestimation of the actual nodal involvement. Thus, it strongly calls for more accurate detection methods such as PET-CT or machine learning-based radiomics. The third limitation was the extent of LND. Although we have established the principles of LND as previously described, the extent of LND was mostly determined by each surgeon. The median retrieval of lymph nodes was 4 in the LND group, which was far from at least six nodes based on the latest guideline. Inadequate LND may lead to misinterpretation of pathological results and affect survival outcomes. Additionally, detailed data on postoperative chemotherapy drugs and doses, radiotherapy dosage and treatment after recurrence were lacking. Last but not least, the study was conducted in three tertiary hepatobiliary centers, which may cause concerns regarding the generalizability of the findings to other settings, particularly those with less specialized expertise in ICC management.
Despite the limitations above, our study focused on patients with clinically node-negative ICC to evaluate the necessity of LND, which was different from previous studies. We discussed the prophylactic LND in various aspects of survival outcomes, accurate N staging and adjuvant therapy. The results suggest that routine LND may not be applied in patients with clinically node-negative ICC. Postoperative adjuvant therapy is recommended to prolong survivals of ICC patients, especially in non-LND individuals. With more accurate preoperative imaging methods, future prospective, multicentered, large-scale studies are expected to confirm the value of LND for patients with clinically node-negative ICC. Also, it is required to further confirm the effect of postoperative adjuvant therapy in different subgroups of ICC patients.
Conclusions
Based on our data, we found that LND did not significantly improve the prognosis of patients with clinically node-negative ICC. Postoperative adjuvant therapy was associated with prolonged survival of ICC patients, especially in non-LND individuals.
Acknowledgments
The authors acknowledge and express their deepest gratitude to the participants of this research.
Financial Disclosure
This research was supported by grants from the National Natural Science Foundation of China (81902379), Chenguang Program of Shanghai Education Development Foundation and Shanghai Municipal Education Commission (21CGA20), and Cultivation Foundation of Renji Hospital (RJPY-LX-011).
Conflict of Interest
The authors declare that they have no conflict of interest.
Informed Consent
The patients provided their written informed consent.
Author Contributions
Conception and design: Meng Sha and Jie Cao. Provision of study materials or patients: Cheng Lin Qin, Jian Zhang and Jie Cao. Collection and assembly of data: Meng Sha and Zhe Li. Data analysis and interpretation: Meng Sha, Chao Fan and Lei Xia. Manuscript writing: all authors.
Data Availability
The datasets analyzed during the current study are available from the corresponding author on reasonable request.
| References | ▴Top |
- Moris D, Palta M, Kim C, Allen PJ, Morse MA, Lidsky ME. Advances in the treatment of intrahepatic cholangiocarcinoma: an overview of the current and future therapeutic landscape for clinicians. CA Cancer J Clin. 2023;73(2):198-222.
doi pubmed - European Association for the Study of the Liver. Electronic address eee, European Association for the Study of the L. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79(1):181-208.
doi pubmed - Hewitt DB, Brown ZJ, Pawlik TM. Surgical management of intrahepatic cholangiocarcinoma. Expert Rev Anticancer Ther. 2022;22(1):27-38.
doi pubmed - Sirica AE, Gores GJ, Groopman JD, Selaru FM, Strazzabosco M, Wei Wang X, Zhu AX. Intrahepatic cholangiocarcinoma: continuing challenges and translational advances. Hepatology. 2019;69(4):1803-1815.
doi pubmed pmc - Sirica AE, Strazzabosco M, Cadamuro M. Intrahepatic cholangiocarcinoma: Morpho-molecular pathology, tumor reactive microenvironment, and malignant progression. Adv Cancer Res. 2021;149:321-387.
doi pubmed pmc - Rizzo A, Brandi G. Neoadjuvant therapy for cholangiocarcinoma: a comprehensive literature review. Cancer Treat Res Commun. 2021;27:100354.
doi pubmed - Guven DC, Sahin TK, Erul E, Rizzo A, Ricci AD, Aksoy S, Yalcin S. The association between albumin levels and survival in patients treated with immune checkpoint inhibitors: A systematic review and meta-analysis. Front Mol Biosci. 2022;9:1039121.
doi pubmed pmc - Rizzo A, Brandi G. Pitfalls, challenges, and updates in adjuvant systemic treatment for resected biliary tract cancer. Expert Rev Gastroenterol Hepatol. 2021;15(5):547-554.
doi pubmed - Rizzo A, Mollica V, Tateo V, Tassinari E, Marchetti A, Rosellini M, De Luca R, et al. Hypertransaminasemia in cancer patients receiving immunotherapy and immune-based combinations: the MOUSEION-05 study. Cancer Immunol Immunother. 2023;72(6):1381-1394.
doi pubmed pmc - Kim SH, Han DH, Choi GH, Choi JS, Kim KS. Extent of lymph node dissection for accurate staging in intrahepatic cholangiocarcinoma. J Gastrointest Surg. 2022;26(1):70-76.
doi pubmed - Zhang XF, Xue F, Dong DH, Weiss M, Popescu I, Marques HP, Aldrighetti L, et al. Number and station of lymph node metastasis after curative-intent resection of intrahepatic cholangiocarcinoma impact prognosis. Ann Surg. 2021;274(6):e1187-e1195.
doi pubmed - Lluis N, Asbun D, Wang JJ, Cao HST, Jimenez RE, Alseidi A, Asbun H. Lymph node dissection in intrahepatic cholangiocarcinoma: a critical and updated review of the literature. J Gastrointest Surg. 2023;27(12):3001-3013.
doi pubmed - Yeow M, Fong KY, Zhao JJ, Tan AYH, Koh YX, Kam JH, Goh BKP, et al. Value of lymph node dissection in intrahepatic cholangiocarcinoma: a systematic review and meta-analysis. HPB (Oxford). 2024;26(2):161-170.
doi pubmed - Hu H, Xu G, Du S, Luo Z, Zhao H, Cai J. The role of lymph node dissection in intrahepatic cholangiocarcinoma: a multicenter retrospective study. BMC Surg. 2021;21(1):359.
doi pubmed pmc - Zhou R, Lu D, Li W, Tan W, Zhu S, Chen X, Min J, et al. Is lymph node dissection necessary for resectable intrahepatic cholangiocarcinoma? A systematic review and meta-analysis. HPB (Oxford). 2019;21(7):784-792.
doi pubmed - Zhu Y, Mao Y, Chen J, Qiu Y, Wang Z, He J. Preoperative computed tomography features of intrahepatic cholangiocarcinoma for predicting lymph node metastasis and overall survival. J Comput Assist Tomogr. 2019;43(5):729-735.
doi pubmed - Zhang S, Huang S, He W, Wei J, Huo L, Jia N, Lin J, et al. Radiomics-based preoperative prediction of lymph node metastasis in intrahepatic cholangiocarcinoma using contrast-enhanced computed tomography. Ann Surg Oncol. 2022;29(11):6786-6799.
doi pubmed - Ji GW, Zhu FP, Zhang YD, Liu XS, Wu FY, Wang K, Xia YX, et al. A radiomics approach to predict lymph node metastasis and clinical outcome of intrahepatic cholangiocarcinoma. Eur Radiol. 2019;29(7):3725-3735.
doi pubmed - Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205-213.
doi pubmed pmc - Yao XI, Wang X, Speicher PJ, Hwang ES, Cheng P, Harpole DH, Berry MF, et al. Reporting and guidelines in propensity score analysis: a systematic review of cancer and cancer surgical studies. J Natl Cancer Inst. 2017;109(8):djw323.
doi pubmed pmc - Mavros MN, Economopoulos KP, Alexiou VG, Pawlik TM. Treatment and prognosis for patients with intrahepatic cholangiocarcinoma: systematic review and meta-analysis. JAMA Surg. 2014;149(6):565-574.
doi pubmed - Jutric Z, Johnston WC, Hoen HM, Newell PH, Cassera MA, Hammill CW, Wolf RF, et al. Impact of lymph node status in patients with intrahepatic cholangiocarcinoma treated by major hepatectomy: a review of the National Cancer Database. HPB (Oxford). 2016;18(1):79-87.
doi pubmed pmc - Umeda Y, Mitsuhashi T, Kojima T, Satoh D, Sui K, Endo Y, Inagaki M, et al. Impact of lymph node dissection on clinical outcomes of intrahepatic cholangiocarcinoma: Inverse probability of treatment weighting with survival analysis. J Hepatobiliary Pancreat Sci. 2022;29(2):217-229.
doi pubmed pmc - Hoyos S, Navas MC, Restrepo JC, Botero RC. Current controversies in cholangiocarcinoma. Biochim Biophys Acta Mol Basis Dis. 2018;1864(4 Pt B):1461-1467.
doi pubmed - Yoh T, Cauchy F, Le Roy B, Seo S, Taura K, Hobeika C, Dokmak S, et al. Prognostic value of lymphadenectomy for long-term outcomes in node-negative intrahepatic cholangiocarcinoma: A multicenter study. Surgery. 2019;166(6):975-982.
doi pubmed - Ke Q, Wang L, Lin Z, Lou J, Zheng S, Bi X, Wang J, et al. Prognostic value of lymph node dissection for intrahepatic cholangiocarcinoma patients with clinically negative lymph node metastasis: a multi-center study from China. Front Oncol. 2021;11:585808.
doi pubmed pmc - Sposito C, Ratti F, Cucchetti A, Ardito F, Ruzzenente A, Di Sandro S, Maspero M, et al. Survival benefit of adequate lymphadenectomy in patients undergoing liver resection for clinically node-negative intrahepatic cholangiocarcinoma. J Hepatol. 2023;78(2):356-363.
doi pubmed - Chen X, Du J, Huang J, Zeng Y, Yuan K. Neoadjuvant and adjuvant therapy in intrahepatic cholangiocarcinoma. J Clin Transl Hepatol. 2022;10(3):553-563.
doi pubmed pmc - Bartsch F, Hahn F, Muller L, Baumgart J, Hoppe-Lotichius M, Kloeckner R, Lang H. Intrahepatic cholangiocarcinoma: Introducing the preoperative prediction score based on preoperative imaging. Hepatobiliary Pancreat Dis Int. 2021;20(3):262-270.
doi pubmed - Adachi T, Eguchi S, Beppu T, Ueno S, Shiraishi M, Okuda K, Yamashita Y, et al. Prognostic impact of preoperative lymph node enlargement in intrahepatic cholangiocarcinoma: a multi-institutional study by the Kyushu study group of liver surgery. Ann Surg Oncol. 2015;22(7):2269-2278.
doi pubmed - Grobmyer SR, Wang L, Gonen M, Fong Y, Klimstra D, D'Angelica M, DeMatteo RP, et al. Perihepatic lymph node assessment in patients undergoing partial hepatectomy for malignancy. Ann Surg. 2006;244(2):260-264.
doi pubmed pmc - Lin Y, Chong H, Song G, Zhang C, Dong L, Aye L, Liang F, et al. The influence of (18)F-fluorodeoxyglucose positron emission tomography/computed tomography on the N- and M-staging and subsequent clinical management of intrahepatic cholangiocarcinoma. Hepatobiliary Surg Nutr. 2022;11(5):684-695.
doi pubmed pmc - Nishioka E, Tsurusaki M, Kozuki R, Im SW, Kono A, Kitajima K, Murakami T, et al. Comparison of conventional imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography in the diagnostic accuracy of staging in patients with intrahepatic cholangiocarcinoma. Diagnostics (Basel). 2022;12(11):2889.
doi pubmed pmc - Xu L, Yang P, Liang W, Liu W, Wang W, Luo C, Wang J, et al. A radiomics approach based on support vector machine using MR images for preoperative lymph node status evaluation in intrahepatic cholangiocarcinoma. Theranostics. 2019;9(18):5374-5385.
doi pubmed pmc - Zhan PC, Yang T, Zhang Y, Liu KY, Li Z, Zhang YY, Liu X, et al. Radiomics using CT images for preoperative prediction of lymph node metastasis in perihilar cholangiocarcinoma: a multi-centric study. Eur Radiol. 2024;34(2):1280-1291.
doi pubmed - Kelley RK, Bridgewater J, Gores GJ, Zhu AX. Systemic therapies for intrahepatic cholangiocarcinoma. J Hepatol. 2020;72(2):353-363.
doi pubmed - Cloyd JM, Pawlik TM. Trends in the use of adjuvant therapy for resected intrahepatic cholangiocarcinoma: getting ahead of the data. Hepatobiliary Surg Nutr. 2021;10(4):515-517.
doi pubmed pmc - Munir MM, Ruff SM, Endo Y, Lima HA, Alaimo L, Moazzam Z, Shaikh C, et al. Does adjuvant therapy benefit low-risk resectable cholangiocarcinoma? An evaluation of the NCCN guidelines. J Gastrointest Surg. 2023;27(3):511-520.
doi pubmed - Liao P, Cao L, Chen H, Pang SZ. Analysis of metastasis and survival between extrahepatic and intrahepatic cholangiocarcinoma: a large population-based study. Medicine (Baltimore). 2021;100(16):e25635.
doi pubmed pmc - Jeong H, Kim KP, Jeong JH, Hwang DW, Lee JH, Kim KH, Moon DB, et al. Adjuvant gemcitabine plus cisplatin versus capecitabine in node-positive extrahepatic cholangiocarcinoma: the STAMP randomized trial. Hepatology. 2023;77(5):1540-1549.
doi pubmed - Shi GM, Huang XY, Wu D, Sun HC, Liang F, Ji Y, Chen Y, et al. Toripalimab combined with lenvatinib and GEMOX is a promising regimen as first-line treatment for advanced intrahepatic cholangiocarcinoma: a single-center, single-arm, phase 2 study. Signal Transduct Target Ther. 2023;8(1):106.
doi pubmed pmc - Wang Z, Zeng T, Li Y, Zhang D, Yuan Z, Huang M, Yang Y, et al. PD-1 inhibitors plus capecitabine as maintenance therapy for advanced intrahepatic cholangiocarcinoma: a case report and review of literature. Front Immunol. 2021;12:799822.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


