| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 5, October 2024, pages 825-836
Simultaneous Determination of Methotrexate Concentrations in Human Plasma and Cerebrospinal Fluid Using Two-Dimensional Liquid Chromatography: Applications in Primary Central Nervous System Lymphoma
Yan Hong Wanga, Qiang Heb, Feng Wangc, Hao Jianga, Jing Shia, Ji Mab, d, Yu Guo Liua, d
aDepartment of Pharmacy, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, China
bDepartment of Lymphology and Hematology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, China
cDepartment of Pharmacy, The Second Xiangya Hospital, Central South University, Changsha 410011, China
dCorresponding Author: Ji Ma, Department of Lymphology and Hematology, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, Shandong Province, China; Yu Guo Liu, Department of Pharmacy, Shandong Cancer Hospital and Institute, Shandong First Medical University and Shandong Academy of Medical Sciences, Jinan 250117, Shandong Province, China
Manuscript submitted July 6, 2024, accepted August 21, 2024, published online September 16, 2024
Short title: Determination of MTX by 2D-LC in PCNSL
doi: https://doi.org/10.14740/wjon1910
| Abstract | ▴Top |
Background: In this study, we aimed to develop a method for the simultaneous quantification of methotrexate (MTX) samples extracted from human plasma and cerebrospinal fluid (CSF), using two-dimensional liquid chromatography (2D-LC). Furthermore, we intended to verify whether intravenous mannitol could increase MTX concentration in the CSF of patients.
Methods: The mobile phase of PUMP1 consisted of 10.0 mmol/L ammonium acetate and acetonitrile. PUMP2 solution consisted of an aqueous solution of 10.0 mmol/L ammonium acetate. The mobile phase of PUMP3 comprised 50.0 mmol/L ammonium acetate and acetonitrile, with a flow rate of 1.0 mL/min.
Results: The developed method was successfully employed to simultaneously determine drug levels in plasma and CSF from the patients treated with MTX. CSF samples were obtained by lumbar puncture 0.5 - 2 h after starting the high-dose methotrexate (HD-MTX) infusion (over 4 h) and immediately before the intrathecal (IT) administration of MTX. Venous blood samples were drawn 4 h after the start of infusion. The calibration curve was linear, with a range of 0.07 - 2.38 µmol/L for CSF samples and a range of 0.11 - 5.51 µmol/L for plasma samples. Precision (> 95%) and accuracy (> 97%) were within the acceptance criteria for each quality control (QC) level. Inter- and intra-day accuracy and precision values met the acceptance criteria for each QC level. The correlation between MTX concentrations in the plasma and CSF was moderate (r = 0.502). No significant difference was observed in MTX concentration in CSF between patients using intravenous mannitol and those not using intravenous mannitol (P = 0.682).
Conclusion: The developed method was useful for therapeutic drug monitoring of MTX and suitable for assessing the risks and benefits of chemotherapy in patients with primary central nervous system lymphoma. Intravenous mannitol did not increase MTX concentration in the CSF of patients.
Keywords: Primary central nervous system lymphoma; Methotrexate; Cerebrospinal fluid; Two-dimensional liquid chromatography
| Introduction | ▴Top |
Primary central nervous system lymphoma (PCNSL), a highly aggressive form of non-Hodgkin lymphoma (NHL), affects only the brain parenchyma, leptomeninges, cerebrospinal fluid (CSF), and vitreoretinal compartment, with no peripheral involvement [1, 2]. Over 95% of PCNSL cases are of diffuse large B-cell lymphoma (DLBCL), which constitutes about 3-4% of all intracranial malignancies [3]. PCNSL is known for its marked aggressiveness and poor prognosis, both of which contribute to dismal clinical outcomes [4]. Untreated PCNSL patients have an overall survival time of just 1.5 months. Despite the effectiveness of chemotherapy, 35-60% of PCNSL patients experience a relapse within 2 years of initial treatment [3, 5]. High-dose methotrexate (HD-MTX) administered intravenously is the standard induction therapy for PCNSL, as it can cross the blood-brain barrier (BBB). Methotrexate (MTX) doses in most regimens typically range from 1 to 8 g/m2 [6]. Recent years have seen significant advances in PCNSL prognosis, largely due to the use of HD-MTX [7]. However, MTX’s poor selectivity harms not only tumor cells but also normal cells, resulting in severe side effects. The kidneys eliminate over 90% of MTX, accounting for most of its total clearance rate. Delayed elimination of MTX can expose patients to serious toxicity risks such as nephrotoxicity, hepatotoxicity, myelosuppression, severe mucositis, pancytopenia, and other adverse effects [8, 9]. These adverse events can lead to prolonged hospital stays, increased economic burdens, delayed chemotherapy, reduced doses, treatment failures, or even death. Safe HD-MTX treatment involves maintaining blood MTX levels within an effective range and rapidly reducing them to a safe range. Implementing prophylactic measures, including fluid hydration, urine alkalization, and leucovorin rescue, is essential [10, 11]. Additionally, therapeutic drug monitoring (TDM) measures plasma MTX concentrations to ensure HD-MTX’s safety and effectiveness [12].
TDM is a clinical discipline focused on developing mechanisms, technologies, methods, and standards for personalized drug therapy. It leverages research outcomes to optimize the rational use of medications in clinical settings. TDM plays a critical role in creating personalized drug delivery plans that consider factors like the drug treatment window [13]. Currently, TDM is commonly used for drugs that show significant variability between and within individuals, and those with narrow, highly toxic therapeutic windows [14]. HD-MTX therapy is highly toxic and shows significant variability in MTX clearance among individuals during each treatment cycle. For safety, MTX concentrations must be monitored at 24, 48, and 72 h after administration to meet specific detection criteria. Specifically, plasma MTX concentrations should not exceed 10, 1, and 0.1 µmol/L at 24, 48, and 72 h, respectively [15]. Although HD-MTX-based therapies are standard for PCNSL induction, their effectiveness is still debated. The blood-brain barrier (BBB) significantly limits drug delivery to brain tissues, restricting chemotherapy’s reach within the central nervous system. However, evidence indicates that the BBB is compromised at PCNSL sites, potentially enhancing drug penetration [16, 17]. Intravenous mannitol, a safe method for disrupting the BBB to increase intracerebral MTX levels, has not yet seen widespread clinical use.
In this study, we aimed to establish a simple and rapid method for simultaneously measuring MTX concentrations in CSF and plasma. We also attempted to prove the correlation between the concentration of MTX in plasma and that in CSF, so as to infer the concentration of MTX in the CSF of patients based on the concentration of MTX in the plasma of patients, avoiding the traumatic damage and economic loss caused by collecting CSF from patients. Additionally, this study also sought to explore the correlation of MTX concentration in the CSF between patients who received intravenous mannitol treatment and those who did not, in order to determine whether intravenous mannitol can enhance the permeability of the BBB to MTX.
| Materials and Methods | ▴Top |
This study protocol was reviewed and approved by Ethics Committee of Shandong Cancer Institute (approval number 2024003025). All the relevant ethical safeguards have been met with the patient.
Reagents and chemicals
The MTX reference material (free base, purity > 99%) was obtained from the National Institute for the Control of Pharmaceutical and Biological Products (Beijing, China). Acetonitrile and methanol were high-performance liquid chromatography (HPLC) grade, and ammonium phosphate, ethylene glycol, and ammonium acetate were analytical grade. All reagents and chemicals were procured from Sinopharm Chemical Reagent Co. Ltd. (Shanghai, China). Distilled water was used throughout the experiments. Blank human plasma, used for setting up the experiments, was obtained from the hospital medical examination center, and CSF was acquired from untreated patients in the Department of Lymphology and Hematology (Shandong Cancer Hospital and Institute, Jinan, China). All patients provided duly signed informed consent forms.
Chromatographic system
The 2D-LC system included a first-dimensional liquid chromatography system (LC1), an interface, and a second-dimensional liquid chromatography system (LC2). LC1 included an LC1 column (first separation column), an autosampler device (SIL-20A, 500-l quantitative loop, Shimadzu; SIL), and a chromatography pump (LC-20ATvp, Shimadzu, Kyoto, Japan; PUMP1). The interface housed two capture columns (A and B), 6- and 10-port switching valves (Rheodyne, Oak Harbor, WA), and an auxiliary high-pressure pump (LC-20ATvp; PUMP2). LC2 consisted of an LC2 column (second separation column), a UV detector (SPD-20A, Shimadzu), a workstation (LC solution ver. 1.26, Shimadzu), and a low-pressure gradient chromatography pump (LC-20ATvp; PUMP3). The system included three columns: an LC1 column (Aston FS SC2, 3.5 × 50 mm, 5 µm, ANAX, Changsha, China), a capture column (Aston SN, 4.6 × 10 mm, 5 µm, ANAX), and an LC2 column (Aston SCX4, 4.6 × 125 mm, 5 µm, ANAX). Other instruments included a Mini-15K micro-high-speed centrifuge (Allsheng Instruments, Hangzhou, China), a GH-202 electronic analytical balance (A&D, Tokyo, Japan), an XW-80 vortex mixer (QITE, Shanghai, China), a medical refrigerator (MEILING, Hefei, China), and a TDZ4-WS low-speed centrifuge (XIANGYI, Xiangtan, China).
PUMP1’s mobile phase consisted of 10.0 mmol/L ammonium acetate and acetonitrile in a 90:10 v/v ratio, with the pH adjusted to 3.8 using acetic acid, and a flow rate of 1.2 mL/min. The PUMP2 solution was an aqueous mix of 10.0 mmol/L ammonium acetate, with the pH adjusted to 3.0 using acetic acid. PUMP3’s mobile phase included 50.0 mmol/L ammonium acetate and acetonitrile in an 82.5:17.5 v/v ratio, pH adjusted to 5.2 with acetic acid, and a flow rate of 1.0 mL/min. The detection wavelength was set at 302 nm. The detector cell temperature was maintained at 40 °C, and the autosampler’s injection volume was 200 µL.
Stock and standard solutions
A mixed solution of methanol and ethylene glycol (3:1, v/v) was used to prepare the standard stock solution of MTX at 23.80 and 55.10 µmol/L. Two groups of working solutions were prepared by serial dilution from the stock solution with 25% ethylene glycol. The first group comprised concentrations of 0.24, 2.38, and 11.90 µmol/L, while the second group comprised concentrations of 1.10, 5.51, and 27.55 µmol/L. All these working solutions were freshly prepared and used for calibration purposes. Calibration concentrations were set at 0.07, 0.15, 0.30, 0.59, 1.19, and 2.38 µmol/L for CSF and 0.11, 0.28, 1.10, 2.76, 3.86, and 5.51 µmol/L for human plasma. They were prepared by spiking blank CSF and plasma with the corresponding working solutions. Internal quality control (QC) samples were similarly prepared at three different concentrations, comprising low-, mid-, and high-level QC samples of 0.24, 0.71, and 1.49 µmol/L for CSF and 0.55, 2.20, and 4.13 µmol/L for plasma, respectively. All stock and working solutions, calibration solutions, and QC samples were stored at -80 °C and brought to ambient temperature before use.
Sample preparation
In the initial step, 200 µL plasma or CSF sample was pipetted into a 1.5 mL polypropylene microtube. Subsequently, 600 µL of an 85:15 (v/v) solution of 6% perchloric acid-methanol was added. The tube was vigorously vortexed for 30 s and centrifuged at 14,500 × g for 5 min at ambient temperature. Finally, the clear supernatant (600 µL) was transferred into a glass injection vial for analysis. Both the blank and patient samples were treated identically.
Clinical samples
All patients underwent an HD-MTX-based chemotherapy regimen, with each HD-MTX treatment administered as a 4-h infusion. CSF and corresponding serum samples were not collected simultaneously. CSF samples were obtained by lumbar puncture 0.5 - 2 h after starting the HD-MTX infusion (over 4 h) and immediately before the intrathecal (IT) administration of MTX. Venous blood samples were drawn 4 h after the start of infusion. Additionally, as part of routine clinical treatment drug monitoring, plasma MTX concentrations were measured at 24, 48, and 72 h after infusion completion. To ensure a steady-state concentration of MTX in the plasma, CSF samples and their corresponding plasma MTX concentrations were included in the data analysis only if blood sampling had been performed less than 5 h after the start of infusion. The availability of the data is determined based on the administration time of MTX and the blood collection time as recorded in the case system.
Validation methodology
Validation procedures employed a method for establishing and validating bioanalysis according to FDA guidelines [18].
| Results | ▴Top |
Method design and development
During optimization, various concentrations of mobile phase were tried in an isocratic and gradient mode. The final selected concentrations were as follows. The mobile phase of LC1 consisted of ammonium acetate and acetonitrile (90: 10, v/v, pH 3.8), with a flow rate of 1.2 mL/min. The mobile phase of LC2 consisted of 50.0 mmol/L ammonium acetate and acetonitrile (82.5:17.5, v/v, pH 5.2), with a flow rate of 1.0 mL/min. In PUMP1, the flow rate had a significant effect on the retention time and peak area. In PUMP3, an increase in the proportion of ammonium acetate increased the retention time but did not significantly increase the peak area. Chromatographic separation in LC1 and LC2 was distinct, with no observed interference (Fig. 1). For the accurate detection of MTX, achieving maximum absorption, and minimizing interference, the wavelength of 302 nm was selected as the absorption spectrum. When samples were treated with perchloric acid and acetonitrile, it was found that the samples treated with perchloric acid were no interference of endogenous components, and the recovery rate of the samples was higher. To enhance the recovery rate of the samples, plasma and CSF samples were treated with perchloric acid. The detector cell operated at a temperature of 40 °C, and the autosampler injection volume was 200 µL. Under these conditions, the chromatographic separation was clear, the MTX peak exhibited a satisfactory shape, and no interference was observed. The analysis time was approximately 7.55 min (Fig. 2).
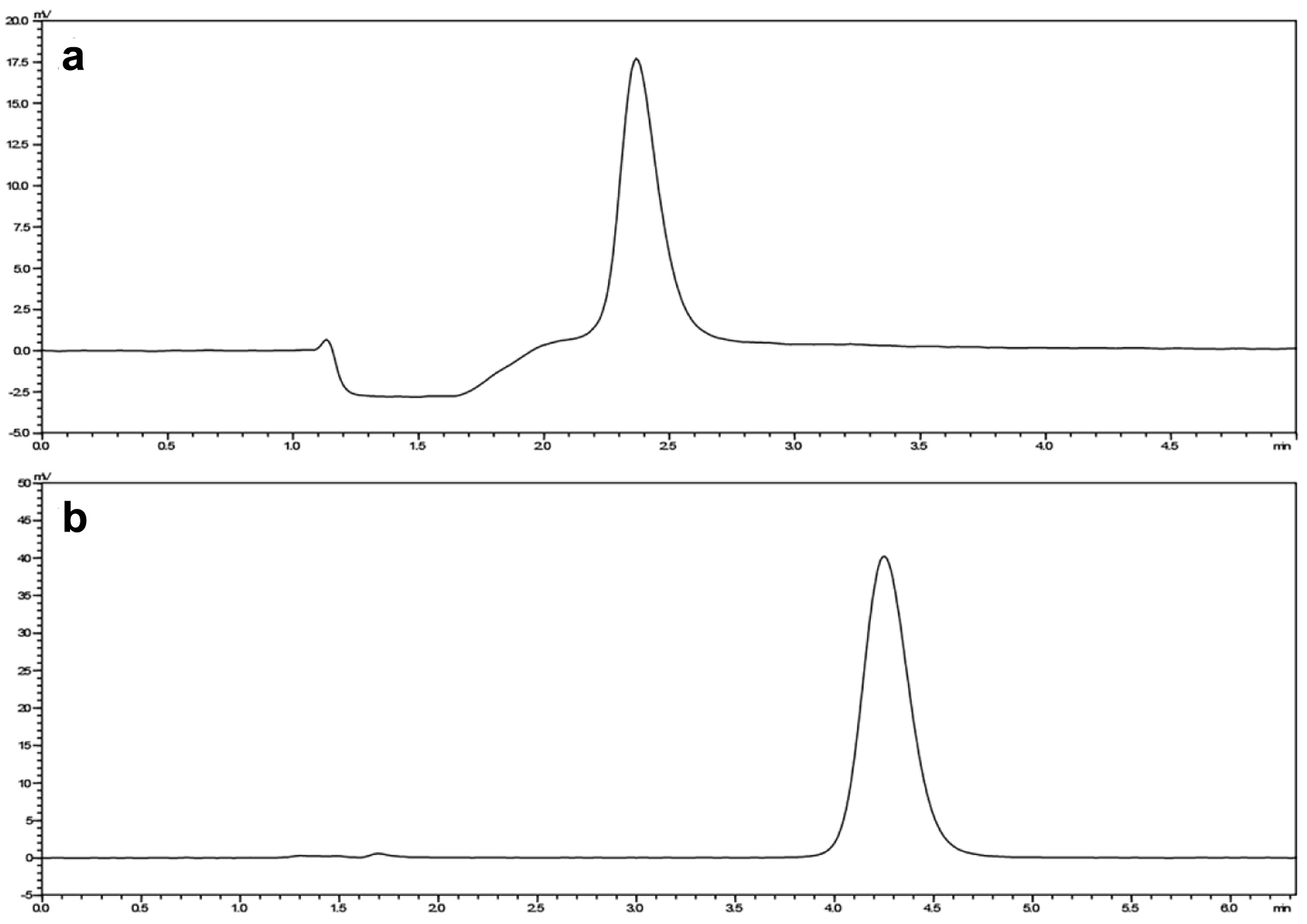 Click for large image | Figure 1. The peak area of MTX LC1 (a) and LC2 (b). LC1: first-dimensional liquid chromatography system; LC2: second-dimensional liquid chromatography system; MTX: methotrexate. |
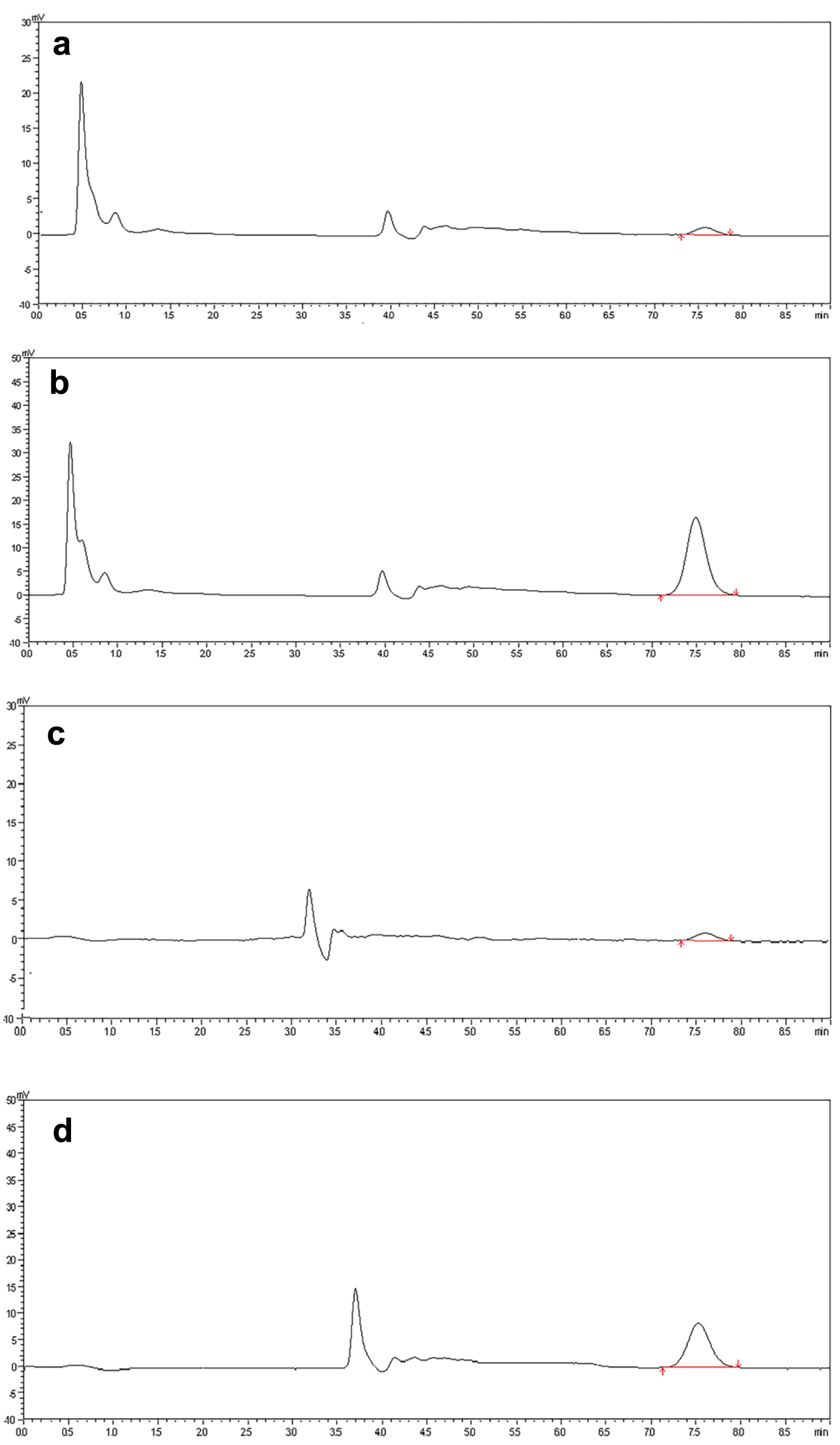 Click for large image | Figure 2. The chromatogram of (a) blank human plasma with MTX 0.05 µmol/L (LLOD) and (b) clinical patient plasma with MTX (5.51 µmol/L). The chromatogram of (c) blank cerebrospinal fluid with MTX 0.05 µmol/L (LLOD) and (d) clinical patient cerebrospinal fluid with MTX (2.38 µmol/L). MTX: methotrexate. |
Method validation
Selectivity
Selectivity was ascertained using six drug-free CSF and plasma samples each, along with spiked CSF samples (at 2.38 µmol/L) and spiked plasma samples (at 5.51 µmol/L). These samples were processed without the inclusion of target compounds. Representative chromatograms are displayed in Figure 3, indicating a satisfactory MTX peak shape, a reasonably steady baseline, and the absence of other peaks.
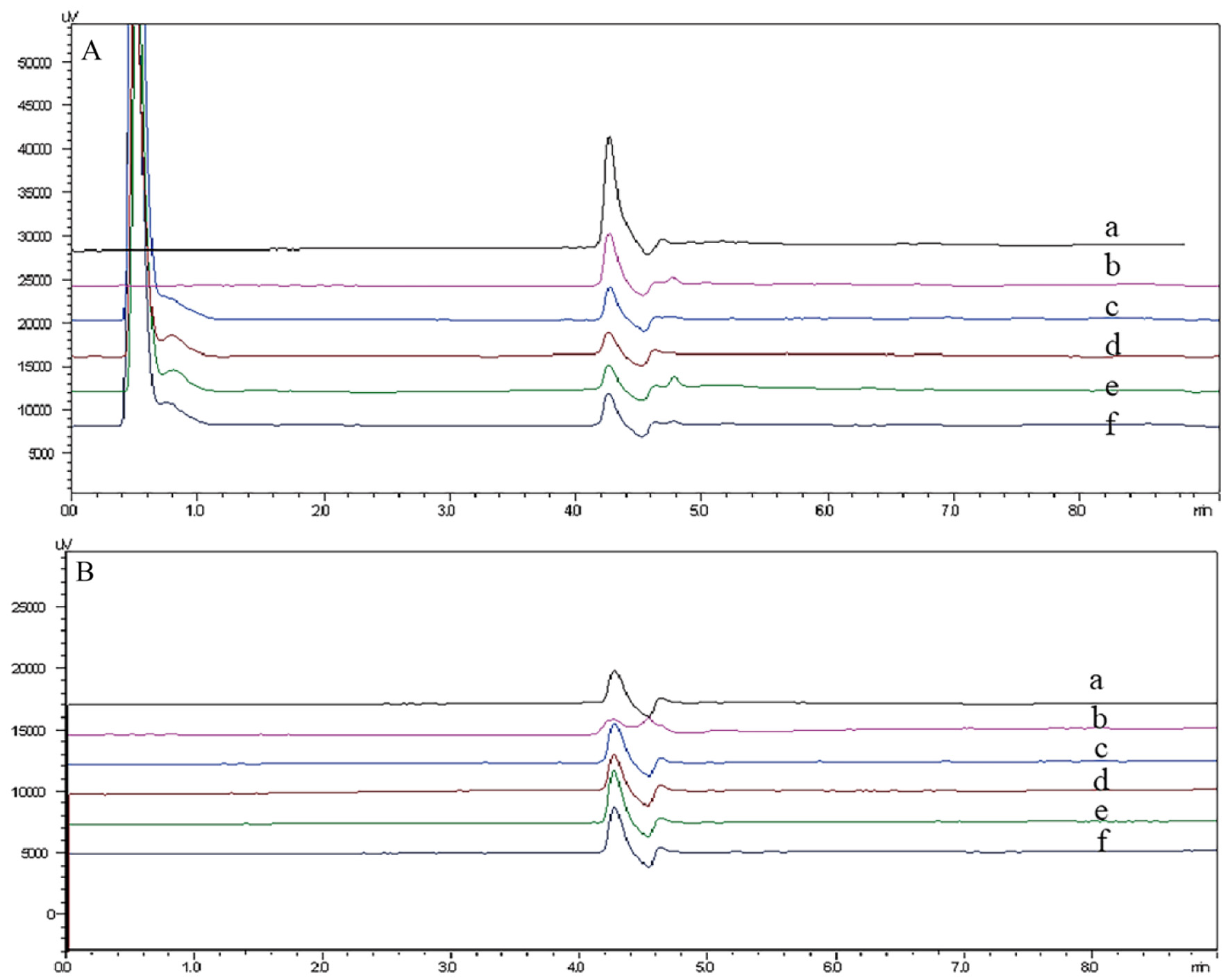 Click for large image | Figure 3. (A) (a) The chromatogram of blank plasma. (b-f) Clinical patient plasma without MTX. (b) Patient 1 drugs: cyclophosphamide, cytarabine, omeprazole, dexamethasone, ondansetron, levocarnitine, methotrexate, and magnesium isoglycyrrhizinate. (c) Patient 2 drugs: vancomycin, caspofungin, spironolactone, lidocaine, reduced glutathione, methylprednisolone, imipenem and cilastatin, voriconazole, and linezolid. (d) Patient 3 drugs: vitamin B6, furosemide, pantoprazole, methotrexate, magnesium isoglycyrrhizinate, vitamin C, tropisetron, rituximab, indomethacin, and promethazine. (e) Patient 4 drugs: cefoperazone and sulbactam, torasemide, pantoprazole, palonosetron, rituximab, promethazine, methotrexate, levoleucovorin calcium, and recombinant human interleukin. (f) Patient 5 drugs: idarubicin hydrochloride, vindesine, sulfate mesna, cyclophosphamide, omeprazole, prednisone acetate, and meropenem. (B) (a) The chromatogram of blank cerebrospinal fluid. (b-f) Clinical patient cerebrospinal fluid without MTX. (b) Patient 1 drugs: cyclophosphamide, cytarabine, omeprazole, dexamethasone, ondansetron, levocarnitine, methotrexate, and magnesium isoglycyrrhizinate. (c) Patient 2 drugs: vancomycin, caspofungin, spironolactone, lidocaine, reduced glutathione, methylprednisolone, imipenem and cilastatin, voriconazole, and linezolid. (d) Patient 3 drugs: vitamin B6, furosemide, pantoprazole, methotrexate, magnesium isoglycyrrhizinate, vitamin C, tropisetron, rituximab, indomethacin, and promethazine. (e) Patient 4 drugs: cefoperazone and sulbactam, torasemide, pantoprazole, palonosetron, rituximab, promethazine, methotrexate, levoleucovorin calcium, and recombinant human interleukin. (f) Patient 5 drugs: idarubicin hydrochloride, vindesine, sulfate mesna, cyclophosphamide, omeprazole, prednisone acetate, and meropenem. MTX: methotrexate. |
Accuracy and precision
The inter- and intra-day precision and accuracy were evaluated across three QC levels (low, mid, and high). In the intra-day accuracy experiments, five replicates of each QC level were assayed in a single run. In the inter-day experiments, three replicates of each QC level were assayed for five consecutive days. The precision and accuracy of the method were expressed as relative standard deviation (RSD, %) and as the percent deviation of measured concentration from the nominal value, respectively. Table 1 summarizes the validation sample data for assay performance. Precision (< 5%) and accuracy (< 3%) were within the acceptance criteria for each QC level. Both inter- and intra-day accuracy and precision values met the acceptance criteria for each QC level.
 Click to view | Table 1. Intra- and Inter-Assay Precision and Accuracy Results of MTX (n = 5) |
Sensitivity and linearity
Six concentration levels of MTX and a blank CSF sample were used to construct a standard curve 1. The calibration curve (n = 6) exhibited linearity within the concentration range of 0.07 - 2.38 µmol/L for MTX (Fig. 4a). The calibration curve equation is presented in Equation (1). Using six concentration levels of MTX and a blank sample, a standard curve 2 was generated. The calibration curve (n = 6) displayed linearity within the concentration range of 0.05 - 5.51 µmol/L for MTX (Fig. 4b). The calibration curve equation is presented in Equation (2). These two curves were observed to coincide, indicating that the method is suitable for the simultaneous determination of MTX concentration in both CSF and plasma. Curve 3 was generated through curve fitting for all samples using Equation (3) (Fig. 4c).
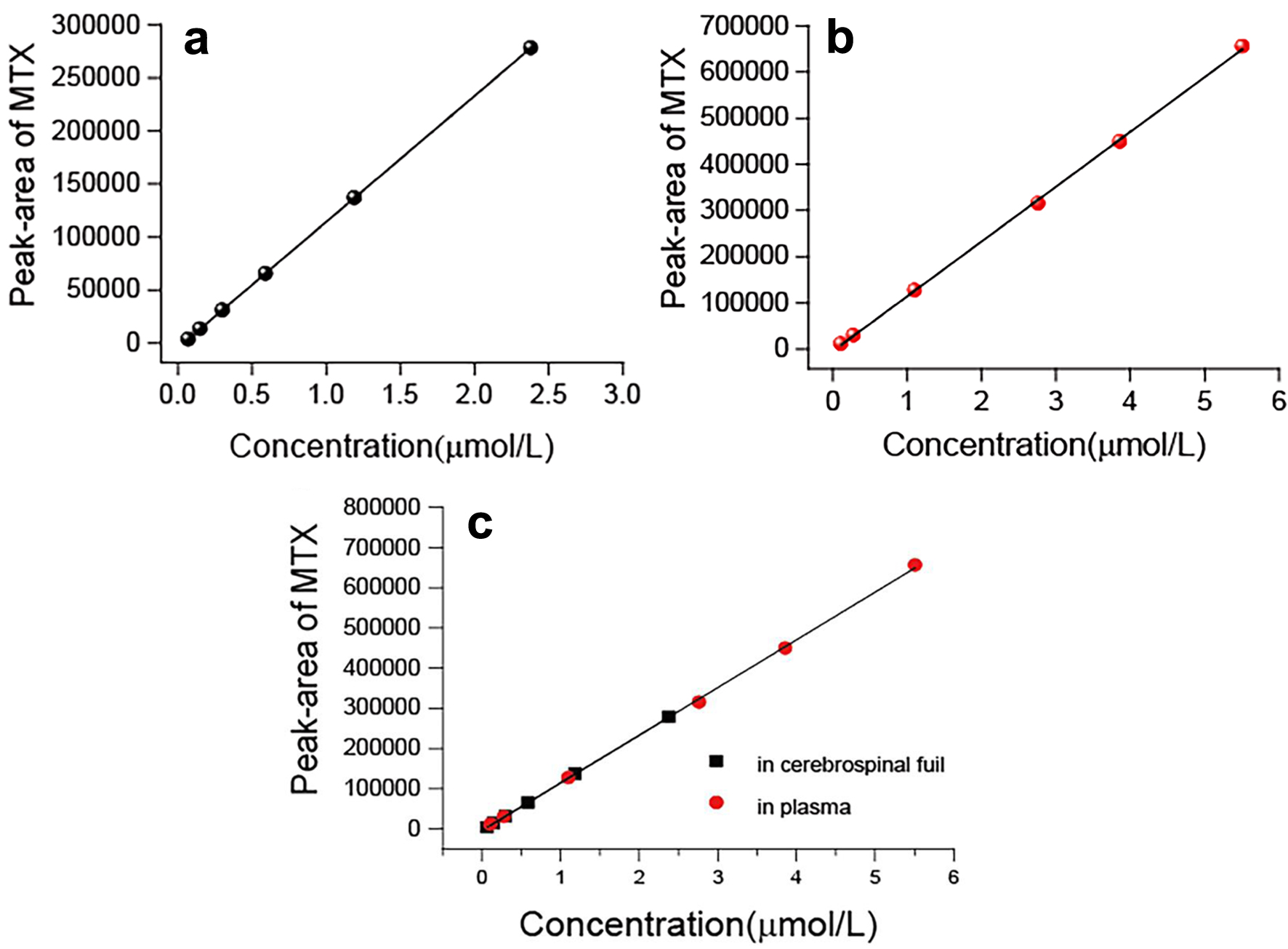 Click for large image | Figure 4. (a) The linearity of the standard curve was observed at MTX concentrations that ranged 0.07 to 2.38 µmol/L (cerebrospinal fluid). (b) The linearity of the standard curve was observed at MTX concentrations that ranged 1.10 to 5.51 µmol/L (human plasma). (c) The linearity of the standard curve was observed at MTX concentrations that ranged 0.07 to 5.51 µmol/L (cerebrospinal fluid and human plasma). MTX: methotrexate. |
In both cases, the lowest limit of quantitation (LLOQ) was 0.05 and 0.07 µmol/L, respectively, with a signal-to-noise ratio of ≥ 10.
Recovery
Extraction recovery was determined by comparing the peak areas of processed low-, mid-, and high-level QC samples (n = 5 for each level) with reference methanol solutions containing the target compound at equivalent concentrations. The extraction recoveries for CSF samples were 100.69±4.04%, 100.85±1.61%, and 99.84±1.46% for the low-, mid-, and high-level QC samples, respectively (Table 2). The extraction recoveries for plasma samples were 99.33±2.12%, 100.55±2.70%, and 99.99±2.90% for the low-, mid-, and high-level QC samples, respectively. This method exhibited a high extraction recovery rate, thereby meeting the requirements of clinical testing.
 Click to view | Table 2. Recovery Results of MTX |
Stability
Stability studies were performed on the low-, mid-, and high-level QC samples. These samples were frozen in a refrigerator (-80 °C) for 12 h and subsequently thawed at room temperature for three freeze-thaw cycles to analyze freeze-thaw stability. For short-term stability testing, samples were placed at room temperature for 6 h, whereas for long-term stability testing, samples were stored at -80 °C for 30 days. The stability results indicated that the level of stability, as denoted by the RSD, was less than 4% (Table 3). MTX remained stable in plasma and CSF samples after three freeze-thaw cycles (-80 °C to room temperature) as well as when stored at room temperature for 6 h and at -80 °C for 30 days. No detrimental effect was observed on the plasma and CSF concentrations of MTX under the studied conditions (Table 3).
 Click to view | Table 3. Results of Stability (n = 5) |
Clinical application
We measured the concentrations of MTX in CSF and plasma from 35 patients and analyzed these two concentrations. The test results are presented in Table 4 and Figure 5. MTX concentrations of 1 µmol/L or greater were detected in 76.47% of CSF samples. In our study, the correlation between MTX concentrations in the plasma and CSF was moderate (r = 0.502). The concentrations of MTX in CSF and plasma exhibited a significant correlation with the dosage of MTX administered (P = 0.0002 and P = 0.0372, respectively). Additionally, we analyzed the difference in MTX concentration in CSF between 15 patients treated with intravenous mannitol and 20 patients not treated with intravenous mannitol, and the results were not significantly different. These results indicated that intravenous mannitol may not increase the concentration of MTX in the CSF of patients.
 Click to view | Table 4. Test Results of Plasma and Cerebrospinal Fluid Concentration of Subjects |
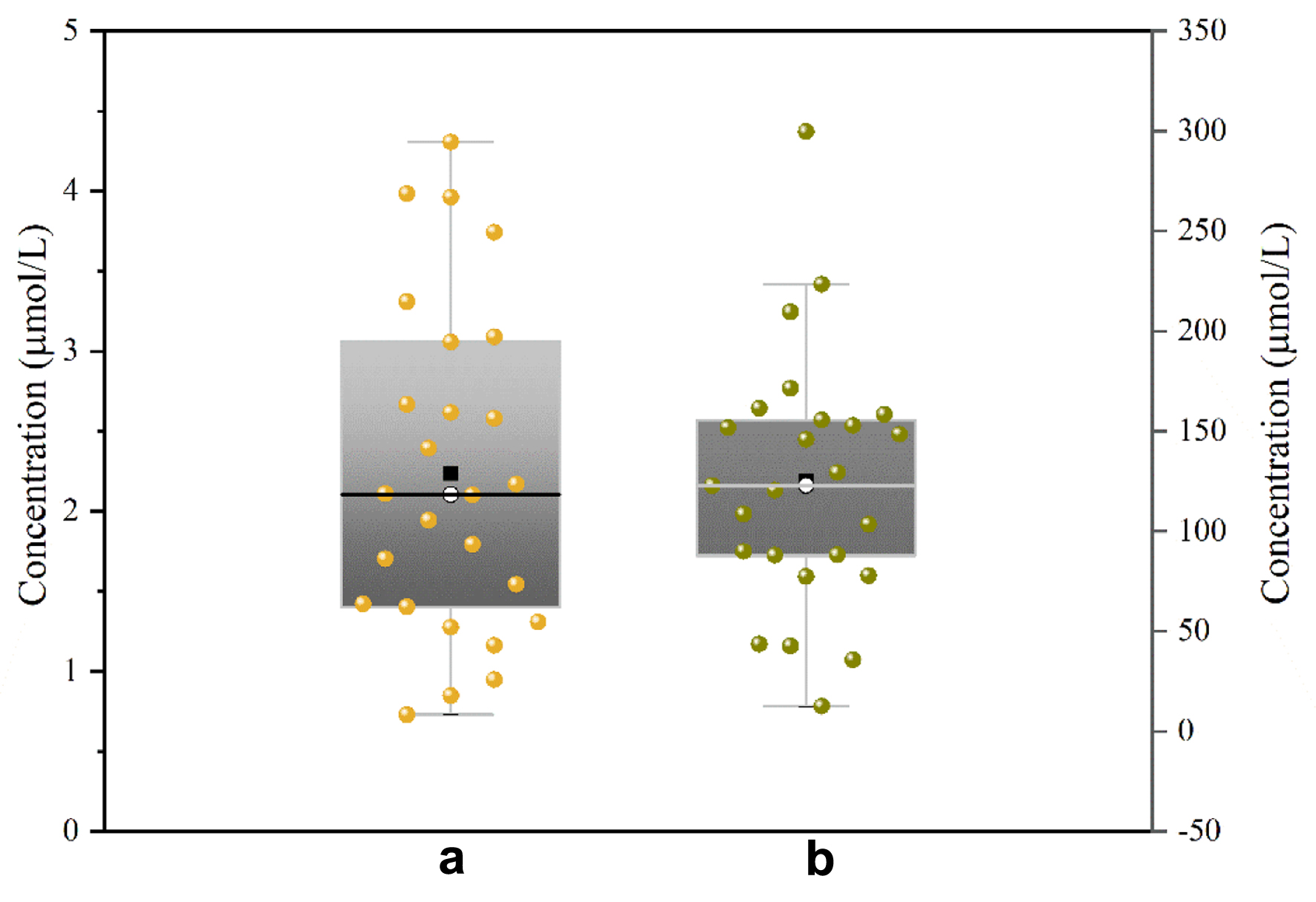 Click for large image | Figure 5. Figure 5. Test results from samples of (a) CSF and (b) plasma. CSF: cerebrospinal fluid. |
| Discussion | ▴Top |
2D-LC is renowned for its superior robustness and real-time performance [19, 20]. In China, more than 200 units use 2D-LC, and its accuracy is widely recognized. A core patent of this new two-dimensional system addresses the challenge of controlled transfer of objects between one and two dimensions (US10859476 B2; JP6639527). The LC1 and LC2 columns of the fully automated 2D-LC used the unique 2D column system (RP1-IEX-RP2), wherein the retention mechanisms of RP1 and IEX differed substantially, leading to strong orthogonality of RP1-IEX. The LC1 column first separated MTX and impurities via an RP separation mechanism, followed by an IEX column segregating MTX and co-eluted components via an ion-exchange separation mechanism. Compounds with a strong ion-exchange capacity, such as MTX, were retained by the IEX column, whereas the co-eluted nonionic and anionic components were removed. This demonstrated the enhanced resolving power and high sensitivity of 2D-LC, making it suitable for determining extremely complex, multi-component mixtures. The new 2D-LC featured dual-level control and hierarchical diagnosis, enabling both positioning research and independent analysis of one-dimensional coarse separated substances. Its capture column not only captured and transferred the analyte but also performed separation, thereby further enhancing the separation ability of the fully automated 2D-LC. Moreover, factors such as laboratory environments (e.g., ambient temperature and uncontrolled humidity state) and sample status (e.g., hemolyzed and high-fat samples) did not significantly influence testing. Changes in ion concentration and pH in the mobile phase, influenced by the proficiency of the operator and column type, did not significantly affect accuracy. All processes were controlled by the time program of the chromatographic workstation, eliminating the need for manual intervention and ensuring high automation and rapid analysis.
Currently, liquid chromatography mass spectrometry (LC-MS)/MS is primarily used for the quantitative analysis of MTX [12, 21]. Despite being highly sensitive and unaffected by interference from MTX metabolites, this method entails expensive instrumentation and complicated operations. In contrast, the 2D-LC method requires only regular maintenance of chromatographic columns, detectors, and chromatographic pumps, without requiring advanced professional knowledge or training. It is simpler for technicians to learn and operate, offering a significant separation, automation, robustness, and real-time performance. In our study, the linear range of MTX was 0.07 - 2.38 µmol/L for CSF samples and 0.11 - 5.51 µmol/L for plasma samples. LLOQ was 0.05 and 0.07 µmol/L, respectively, and the retention time was 7.55 min. Although still inferior to MS in terms of anti-interference capability, sensitivity, and simultaneous determination of various components, the detection sensitivity of this assay was adequate for performing TDM in PCNSL patients.
Limitations
Our study presents several limitations. First, the analysis relied on routine monitoring data of MTX. Additionally, we did not collect numerous covariates, including genetic polymorphisms, hydration volume, alkalization volume, and the calcium folinate rescue regimen, precluding multivariate analyses that could have identified predictors of outcomes and confounding variables. Second, this is a single-center retrospective study, which may introduce multiple biases. Third, obtaining an adequate number of CSF samples is necessary, and the procedure itself is challenging. Among the CSF samples collected, only those obtained 0.5 - 2 h after the initiation of the HD-MTX infusion were selected for analysis. Therefore, whether all samples reached steady-state concentration in the CSF remains uncertain.
Conclusion
In this study, we developed a robust 2D-LC method with enhanced resolving power and high sensitivity, suitable for determining extremely complex, multi-component mixtures. This method can simultaneously and accurately determine the total and free concentrations of MTX in both CSF and plasma. It was successfully applied to analyze plasma and CSF levels in patients with PCNSL. There are no significant differences between patients treated with intravenous mannitol and patients not treated with intravenous mannitol. However, this conclusion may be limited by the small number of samples we collected. Collecting CSF samples poses potential hazards and presents additional challenges for clinicians. We will continue our study and collect a sufficient number of samples to further explore the effect of intravenous mannitol on MTX across the BBB.
Acknowledgments
We thank Qingchen Zhuang for their outstanding assistance in the collection and management of plasma samples and clinical data.
Financial Disclosure
The authors gratefully acknowledge the financial support of the Natural Science Foundation of Shandong Province (ZR2021MH115), CSCO Clinical Oncology Research Foundation-Hengrui Tumor Research Fund Project (Y-HR2020QN-0175 and Y-SY201901-0011) and Wu Jieping Medical Foundation Clinical Research Grant Program (320.6750.2021-02-40).
Conflict of Interest
The authors have no conflict of interest to declare.
Informed Consent
All patients signed informed consent.
Author Contributions
Yan Hong Wang tested the samples and is the main contributor to the manuscript. Qiang He mainly operated the experiment. Wang Feng provided the technical support. Jing Shi completed part of the experiment. Hao Jiang analyzed and screened the patient’s data. Ji Ma and Yu Guo Liu designed the experiment. All the authors have read and approved the final manuscript.
Data Availability
All data generated or analyzed during this study are included in this published article.
Abbreviations
MTX: methotrexate; CSF: cerebrospinal fluid; 2D-LC: two-dimensional liquid chromatography; QC: quality control; PCNSL: primary central nervous system lymphoma; DLBCL: diffuse large B-cell lymphoma; HD-MTX: high-dose methotrexate; BBB: blood-brain barrier; TDM: therapeutic drug monitoring; LC1: first-dimensional liquid chromatography system; LC2: second-dimensional liquid chromatography system; IT: immediately before the intrathecal; HPLC: high-performance liquid chromatography; LC-MS: liquid chromatography mass spectrometry
| References | ▴Top |
- Low S, Han CH, Batchelor TT. Primary central nervous system lymphoma. Ther Adv Neurol Disord. 2018;11:1756286418793562.
doi pubmed pmc - Schaff LR, Grommes C. Primary central nervous system lymphoma. Blood. 2022;140(9):971-979.
doi pubmed pmc - Villanueva G, Guscott M, Schaiquevich P, Sampor C, Combs R, Tentoni N, Hwang M, et al. A systematic review of high-dose methotrexate for adults with primary central nervous system lymphoma. Cancers (Basel). 2023;15(5):1459.
doi pubmed pmc - Chen C, Sun P, Cui J, Yan S, Chen H, Xia Y, Bi X, et al. High-dose Methotrexate plus temozolomide with or without rituximab in patients with untreated primary central nervous system lymphoma: A retrospective study from China. Cancer Med. 2019;8(4):1359-1367.
doi pubmed pmc - Shah T, Venur VA. Central nervous system lymphoma. Semin Neurol. 2023;43(6):825-832.
doi pubmed - Taylor ZL, Mizuno T, Punt NC, Baskaran B, Navarro Sainz A, Shuman W, Felicelli N, et al. MTXPK.org: a clinical decision support tool evaluating high-dose methotrexate pharmacokinetics to inform post-infusion care and use of glucarpidase. Clin Pharmacol Ther. 2020;108(3):635-643.
doi pubmed pmc - Calimeri T, Steffanoni S, Gagliardi F, Chiara A, Ferreri AJM. How we treat primary central nervous system lymphoma. ESMO Open. 2021;6(4):100213.
doi pubmed pmc - Martinez D, Muhrez K, Woillard JB, Berthelot A, Gyan E, Choquet S, Andres CR, et al. Endogenous metabolites-mediated communication between OAT1/OAT3 and OATP1B1 may explain the association between SLCO1B1 SNPs and methotrexate toxicity. Clin Pharmacol Ther. 2018;104(4):687-698.
doi pubmed - Wang SF, Huang KW, Chou YC, Lee HC, Wu PK, Chen WM, Hung GY, et al. Effect of co-medications and potential risk factors of high-dose methotrexate-mediated acute hepatotoxicity in patients with osteosarcoma. Cancer Med. 2023;12(11):12354-12364.
doi pubmed pmc - Yin T, Liang H, Huang Q, Zhou B, Tang M, Lou J, Xiang D. A Survey of Therapeutic Drug Monitoring Status in China. Ther Drug Monit. 2023;45(2):151-158.
doi pubmed pmc - Sheng Y, Zhou B. High-throughput determination of vancomycin in human plasma by a cost-effective system of two-dimensional liquid chromatography. J Chromatogr A. 2017;1499:48-56.
doi pubmed - Briki M, Murisier A, Guidi M, Seydoux C, Buclin T, Marzolini C, Girardin FR, et al. Liquid chromatography coupled to tandem mass spectrometry (LC-MS/MS) methods for the therapeutic drug monitoring of cytotoxic anticancer drugs: An update. J Chromatogr B Analyt Technol Biomed Life Sci. 2024;1236:124039.
doi pubmed - Sample collection, transport, and storage. Fundamentals of Analytical Toxicology. 2020. p. 23-51.
- Smita P, Narayan PA, J K, Gaurav P. Therapeutic drug monitoring for cytotoxic anticancer drugs: Principles and evidence-based practices. Front Oncol. 2022;12:1015200.
doi pubmed pmc - Mosleh E, Snyder S, Wu N, Willis DN, Malone R, Hayashi RJ. Factors influencing delayed clearance of high dose methotrexate (HDMTX) in pediatric, adolescent, and young adult oncology patients. Front Oncol. 2023;13:1280587.
doi pubmed pmc - Joshi S, Ergin A, Wang M, Reif R, Zhang J, Bruce JN, Bigio IJ. Inconsistent blood brain barrier disruption by intraarterial mannitol in rabbits: implications for chemotherapy. J Neurooncol. 2011;104(1):11-19.
doi pubmed pmc - Rapoport SI. Advances in osmotic opening of the blood-brain barrier to enhance CNS chemotherapy. Expert Opin Investig Drugs. 2001;10(10):1809-1818.
doi pubmed - Bioanalytical Method Validation Guidance for Industry.
- Yu Y, Shi J, Wang F, Tang XH, Liu YG. Quantification of the plasma concentration of apatinib by 2-dimensional liquid chromatography. Ther Drug Monit. 2019;41(4):489-496.
doi pubmed - Li X, Wang F, Xu B, Yu X, Yang Y, Zhang L, Li H. Determination of the free and total concentrations of vancomycin by two-dimensional liquid chromatography and its application in elderly patients. J Chromatogr B Analyt Technol Biomed Life Sci. 2014;969:181-189.
doi pubmed - Li X, Liu Z, Li Z, Xiong X, Zhang X, Yang C, Zhao L, et al. A simple, rapid and sensitive HILIC LC-MS/MS method for simultaneous determination of 16 purine metabolites in plasma and urine. Talanta. 2024;267:125171.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


