| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 15, Number 5, October 2024, pages 844-850
Secondary Hemophagocytic Lymphohistiocytosis Following Dostarlimab Treatment in a Patient With Metastatic Endometrial Cancer
Emilie Rolleta, Sebastien Puigrenierb, Abeer Najema, Benoit Desgrousilliersa, Pierre Rivierea, c
aMedical Oncology Department, Boulogne-sur-Mer Hospital, Boulogne-sur-Mer, France
bNephrology and Internal Medicine Department, Boulogne-sur-Mer Hospital, Boulogne-sur-Mer, France
cCorresponding Author: Pierre Riviere, Medical Oncology Department, Boulogne-sur-Mer Hospital, 62200 Boulogne-sur-Mer, France
Manuscript submitted July 19, 2024, accepted July 27, 2024, published online August 10, 2024
Short title: Secondary HLH After Dostarlimab Treatment
doi: https://doi.org/10.14740/wjon1917
| Abstract | ▴Top |
Immunotherapy is a rapidly expanding cancer treatment strategy. Dostarlimab is administered as the first-line treatment for metastatic endometrial cancer in combination with chemotherapy. Herein, we describe the case of a 72-year-old female patient who developed hemophagocytic lymphohistiocytosis after receiving a single dose of 500 mg of dostarlimab. The patient’s clinical outcome improved after treatment with ruxolitinib and corticosteroids. Oncological treatment was resumed in combination with chemotherapy alone.
Keywords: Immunotherapy; Endometrial cancer; Hemophagocytic lymphohistiocytosis; Adverse effect; Corticosteroids; Ruxolitinib
| Introduction | ▴Top |
Immune checkpoint inhibitors (ICIs) are a class of immunotherapeutic agents whose use is rapidly growing in cancer treatment. Several molecules are currently approved: pembrolizumab and nivolumab, which are anti-programmed cell death protein 1 (PD1); ipilimumab, an anti-cytotoxic T-lymphocyte-associated protein-4; and atezolizumab, an anti-programmed cell death protein ligand 1. Dostarlimab, similar to pembrolizumab, is an anti-PD1 molecule that helps restore immune system activity and initiates an anti-tumor response. Early access to dostarlimab was granted in France by the Health Authority in September 2023 for first-line metastatic endometrial carcinomas associated with a combination of platinum-based chemotherapy [1].
Macrophage activation syndrome, or hemophagocytic lymphohistiocytosis (HLH), is characterized by inappropriate hyperactivation of the immune system, involving aberrant activation of lymphocytes and macrophages and increased production of proinflammatory cytokines (Fig. 1) [2]. There are two types of HLH: primary and secondary. Most secondary forms have infectious, autoimmune, or iatrogenic etiologies. The diagnosis requires the presence of five of the eight criteria (fever; splenomegaly; bicytopenia; hypertriglyceridemia or hypofibrinogenemia; hemophagocytosis, ferritin > 500 µg/L; low/absent natural killer (NK)-cell activity; and soluble CD25 elevation) [3]. The prognosis can quickly become severe, and mortality rates are often high [4]. Unfortunately, clinical heterogeneity often leads to delays in treatment initiation, which is a therapeutic emergency that requires etiological treatment combined with immune-targeted treatment to control inflammation [5]. Furthermore, its pathophysiology remains poorly understood, and the low incidence of this syndrome explains the lack of standardized treatment [4].
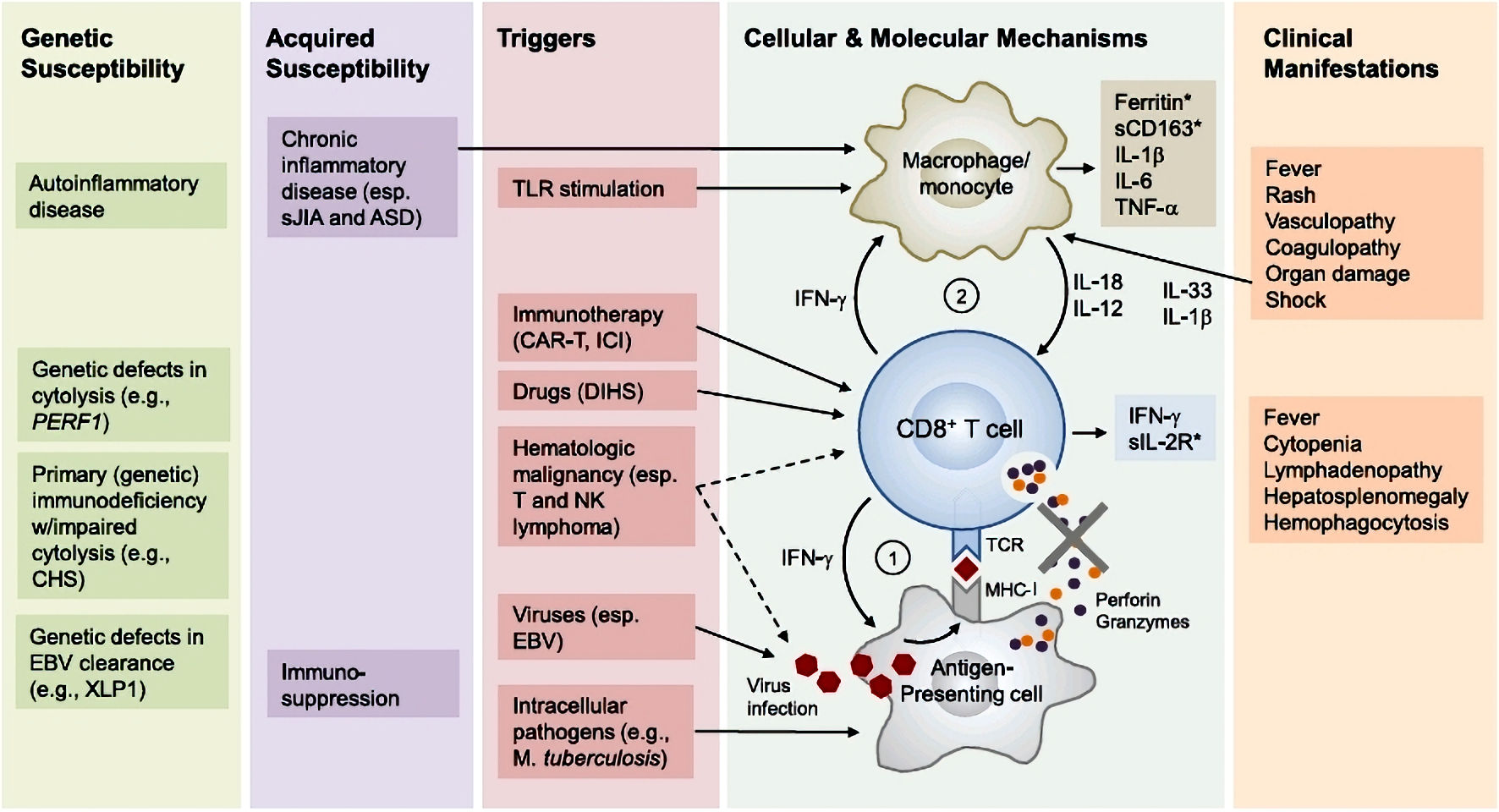 Click for large image | Figure 1. Pathophysiology of hemophagocytic lymphohistiocytosis (adapted from [2]). Primary HLH involves susceptibility genes necessary for the functioning of the immune system, whether it be related to autoinflammatory diseases or a deficiency in clearing certain viruses. Additionally, acquired susceptibility can play a role. The secondary mechanism is triggered by factors such as a viral (EBV) or bacterial (Mycobacterium tuberculosis) infection, the use of an immune checkpoint inhibitor, or a malignant hematopathy. Subsequently, there is an inflammation cascade involving antigen-presenting cells and inflammatory cytokines such as IFN-γ or IL-12, leading to hyperactivation of cytotoxic T lymphocytes and macrophages. This hyperactivation is responsible for the various described symptoms. Notably, the hyperactivation of immune cells involves the tyrosine kinases JAK 1 and 2 at the intracellular level. ASD: autoimmune systemic disease; CART: chimeric antigen receptor T-cell therapy; CD163: cluster of differentiation 163; CHS: Chediak-Higashi syndrome; DHS: drug hypersensitivity syndrome; EBV: Epstein-Barr virus; ICI: immune checkpoint inhibitor; IFN-γ: interferon gamma; IL: interleukin; MHC-I: major histocompatibility complex class I; NK: natural killer; sIL-2R: soluble interleukin-2 receptor; TCR: T-cell receptor; TLR: Toll-like receptor; TNF-α: tumor necrosis factor alpha; XLP1: X-linked lymphoproliferative disease 1. |
HLH is a rare adverse effect associated with ICI treatment. The incidence reported in observational and retrospective studies is < 0.1% [6]. Although several clinical HLH cases associated with treatment with ICIs such as nivolumab, pembrolizumab, or ipilimumab have been described in the literature, to the best of our knowledge, no clinical cases associated with dostarlimab treatment have been previously described [7].
Here, we describe the case of a patient who developed HLH after a single injection of dostarlimab as part of the treatment for metastatic endometrial cancer.
| Case Report | ▴Top |
Investigations
Initial presentation
1) History and clinical findings
A 72-year-old female patient presented to the emergency department with a febrile, maculopapular, morbilliform skin rash and deterioration of her overall condition, along with a non-productive cough (Fig. 2). She had a history of hypertension and a skin allergy to cotrimoxazole. She had been undergoing treatment for high-grade serous endometrial carcinoma with metastases to the lymph nodes and bones for the past 6 months. The tumor profile comprised non-mutated BRCA, proficient mismatch repair, and microsatellite instability (MSI), making the patient eligible for early access treatment with dostarlimab. She had received her first cycle of carboplatin (AUC5), paclitaxel (175 mg/m2), and dostarlimab (500 mg) 14 days prior, with an elevated C-reactive protein (CRP) level of 164 mg/L before treatment due to a high tumor burden. She was admitted to the oncology department for further investigation.
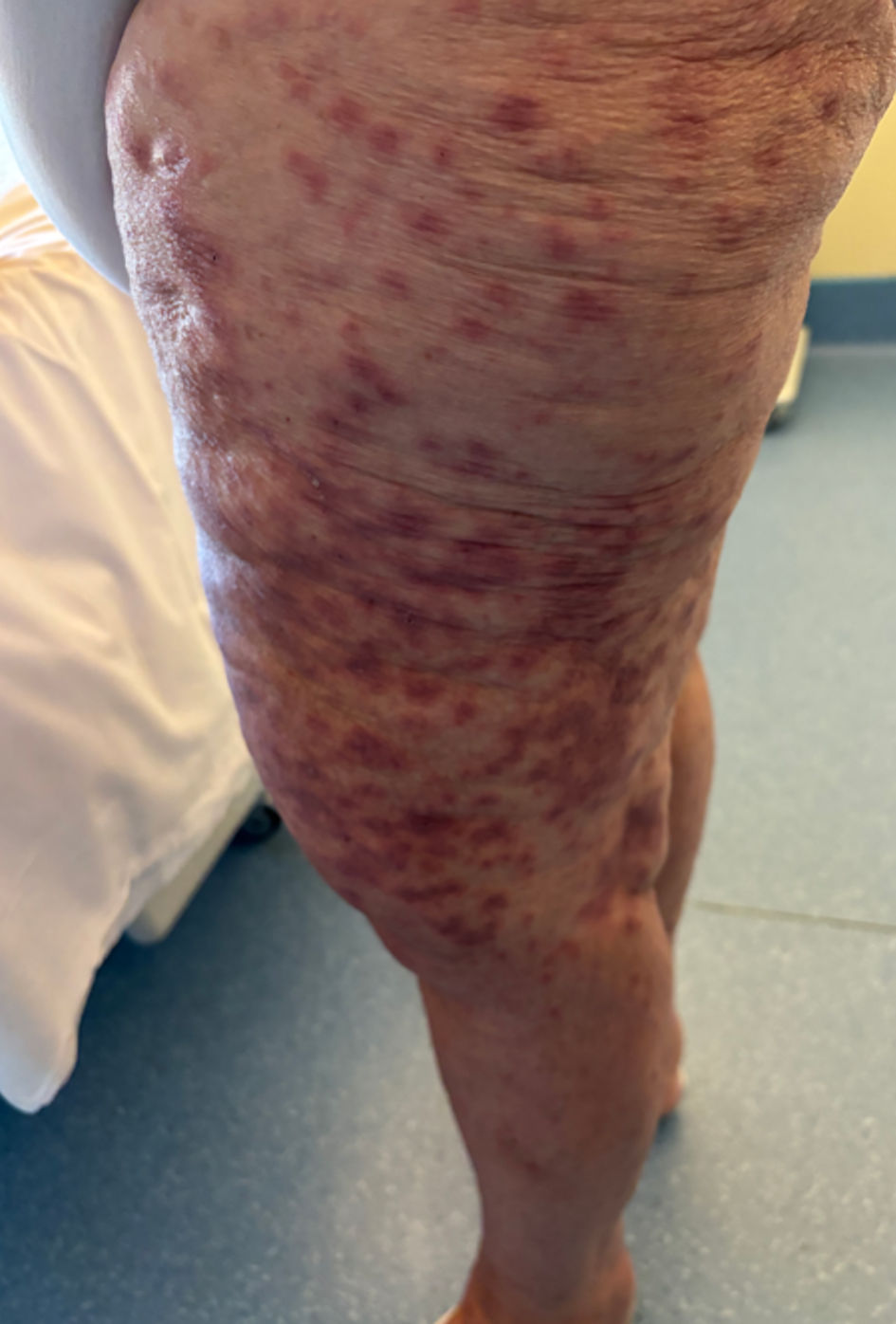 Click for large image | Figure 2. Diffuse morbilliform rash, appearing 14 days after the first treatment. |
2) Laboratory findings
The biological assessment did not show any sign of hemolysis but revealed a biological inflammatory syndrome with a CRP level of 81 mg/L and an unusually high ferritin level of 6,254 ng/mL. A complete blood count showed bicytopenia with anemia at 9.1 g/dL and thrombocytopenia at 109 g/L. A dermatological opinion was also obtained. Three diagnoses were considered for this study. The first was a viral infection, the second was a paraneoplastic rash, and the third was an iatrogenic skin rash induced by the recent introduction of chemotherapy and immunotherapy. Viral serologies were negative for human immunodeficiency virus, hepatitis B virus, and hepatitis C virus. However, the results indicated a past infection with cytomegalovirus (CMV), Epstein-Barr virus (EBV), and parvovirus B19. The blood polymerase chain reaction (PCR) was positive at 3.41 log for EBV, while it was negative for CMV and parvovirus B19. Nasopharyngeal reverse transcription-PCR was negative for influenza A and B viruses, as well as for severe acute respiratory syndrome coronavirus 2. However, nasopharyngeal PCR was positive for respiratory syncytial virus (RSV). Blood cultures were negative. Urinalysis revealed Escherichia coli bacteriuria at 106 CFU/mL with leukocytosis.
3) Imaging findings
An enhanced thoraco-abdominopelvic scan showed only slight thickening of the right pyelocalyceal walls, suggesting pyelonephritis in the context of a positive urine culture.
4) Working diagnosis and initial treatment
A diagnosis of viral skin rash with RSV infection along with pyelonephritis was made, and the patient was treated with cefotaxime, followed by amoxicillin and a course of dermocorticoides. The patient showed partial improvement in fatigue and complete resolution of the skin rash under dermocorticoid treatment. The patient was discharged, and amoxicillin therapy of 2 g three times a day was continued for 7 days. However, thrombocytopenia with a nadir of 93 × 109/L improved with antibiotic therapy, and the inflammatory syndrome persisted (CRP level of 284 mg/L and ferritin level of > 16,500 ng/mL).
Second presentation
1) Clinical findings
The patient returned to our department 1 week after discharge for a second course of oncological treatment. At that time, she presented with significant fatigue and a fever of 38 °C. Clinical examination did not reveal any infectious foci, and there was no recurrence of the skin rash after local corticosteroid treatment.
2) Laboratory findings
Laboratory tests showed signs of inflammation with an elevated white blood cell count, a CRP level of 264 mg/L, and persistently low levels of both red blood cells (hemoglobin, 10.8 g/dL) and platelets (thrombocytopenia: 89 × 109/L). Liver function tests indicated cytolysis, with aspartate aminotransferase and alanine aminotransferase levels three times higher than normal. Blood culture and urine test results were negative for microorganisms.
3) Imaging findings
Thoraco-abdominopelvic computed tomography (CT) revealed bilateral segmental pulmonary embolism and predominantly left-sided pleural effusions with passive collapse of the lung tissue.
4) Working diagnosis and treatment
Owing to suspected pneumonia in a patient recently treated with cefotaxime, treatment with tazocillin was initiated, along with therapeutic anticoagulation using tinzaparin. The following day, laboratory results revealed elevated ferritin and triglyceride levels and initially normal fibrinogen levels. Bone marrow examination revealed hemophagocytosis with no abnormal cell findings or erythroblastic lineage. Further investigations were conducted to determine the underlying causes, considering infectious, neoplastic, and iatrogenic possibilities.
5) Further imaging findings
Evaluation of oncological disease by positron emission tomography (PET) showed regression in the supra-diaphragmatic lymph nodes, a stable morphometabolic aspect of the uterine lesion, diffuse hypermetabolic splenomegaly, and discrete hypermetabolism of bilateral postero-basal lung consolidations suggestive of bilateral atelectasis associated with bilateral pleural effusions. This result did not favor a paraneoplastic etiology.
The search for an infectious etiology using cardiac ultrasonography revealed no images suggestive of infective endocarditis. The PET/CT results initially indicated a possible infectious etiology for bilateral pleuropneumopathy.
6) Clinical course
Two days later, the patient developed acute respiratory distress requiring oxygen. Arterial blood gas analysis revealed respiratory alkalosis, and CT angiography confirmed bilateral pleural effusions associated with bilateral atelectasis (Fig. 3). We added levofloxacin to the antibiotic regimen to treat infections caused by intracellular microorganisms and Legionella.
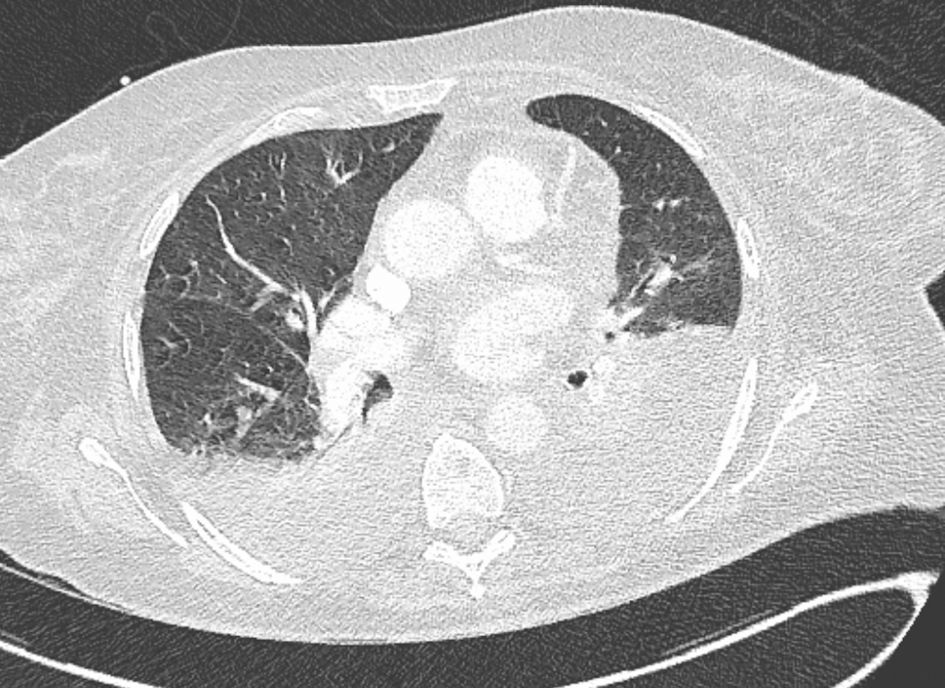 Click for large image | Figure 3. Chest CT revealed bilateral pleural effusions associated with right lower lobe atelectasis. CT: computed tomography. |
7) Further laboratory findings
The infectious workup revealed no catheter infection, no bacteremia, a sterile urine culture, and no pathogenic microorganisms in the sputum. Pleural fluid analysis revealed citrine fluid with a normal cellular formula. The bacterial and mycological cultures were negative, acid-fast bacilli were absent on direct examination, and mycobacterial cultures were negative. PCR test results for Mycoplasma pneumoniae (M. pneumoniae) and Chlamydia pneumoniae (C. pneumoniae) were negative, as was the result for bacterial genome detection of the puncture fluid. A mycobacterial search of the bone marrow was negative upon direct examination and culture. Serological test results for syphilis, Coxiella burnetii, M. pneumoniae, and C. pneumoniae were negative. Nasopharyngeal PCR for M. pneumoniae and C. pneumoniae yielded negative results. Leishmaniasis was ruled out based on a negative blood PCR and serology. Fungal assessments (beta-D-glucan and Aspergillus serology) were negative. Serological test results for CMV, EBV, and parvovirus B19 were negative. Measles and rubella tests confirmed vaccination. Serologies for human herpesvirus (HHV)8, HHV6, and herpes simplex virus type 1 (HSV-1) and 2 showed no recent infection. Viral loads by PCR for HHV6, HHV8, HSV-1 and 2, CMV, and parvovirus B19 tested negative. The EBV load was very weakly positive, unquantifiable, and did not suggest reactivation. The comprehensive infection workup, given the poor response to broad-spectrum antibiotics, ruled out an infectious cause.
Immunoglobulin (Ig) A, IgG, and IgM levels were normal. Serum protein electrophoresis revealed a monoclonal band of gamma globulins with traces of IgG lambda. The CH50, C3, and C4 levels were normal. Antinuclear antibodies were positive but not significant. Anti-neutrophil cytoplasmic antibodies were negative. Angiotensin-converting enzyme and rheumatoid factor levels were within normal limits. Lymphocytic immunophenotyping showed global lymphopenia affecting all studied cellular compartments. Glycosylated ferritin was 7%, although the patient’s age and absence of other clinical signs, such as arthralgia and rashes during febrile episodes, did not support a diagnosis of Still’s disease. Therefore, an autoimmune etiology was not considered to be the cause of the clinical presentation.
8) Further clinical course
Biologically, thrombocytopenia worsened to 13 × 109/L, and anemia necessitated multiple platelet and red blood cell transfusions. Treatment for bilateral pulmonary embolism was discontinued because of thrombocytopenia. Further assessment included venous Doppler ultrasonography of the lower limbs, which revealed bilateral deep vein thrombosis of the common femoral veins and thrombosis of the left soleal vein. A vena cava filter was used in this study. Curative anticoagulation was resumed when the platelet level reached > 50 × 109/L. The triglyceride level peaked at 4.44 g/L, and the fibrinogen level decreased to 1.42 g/L (Fig. 4).
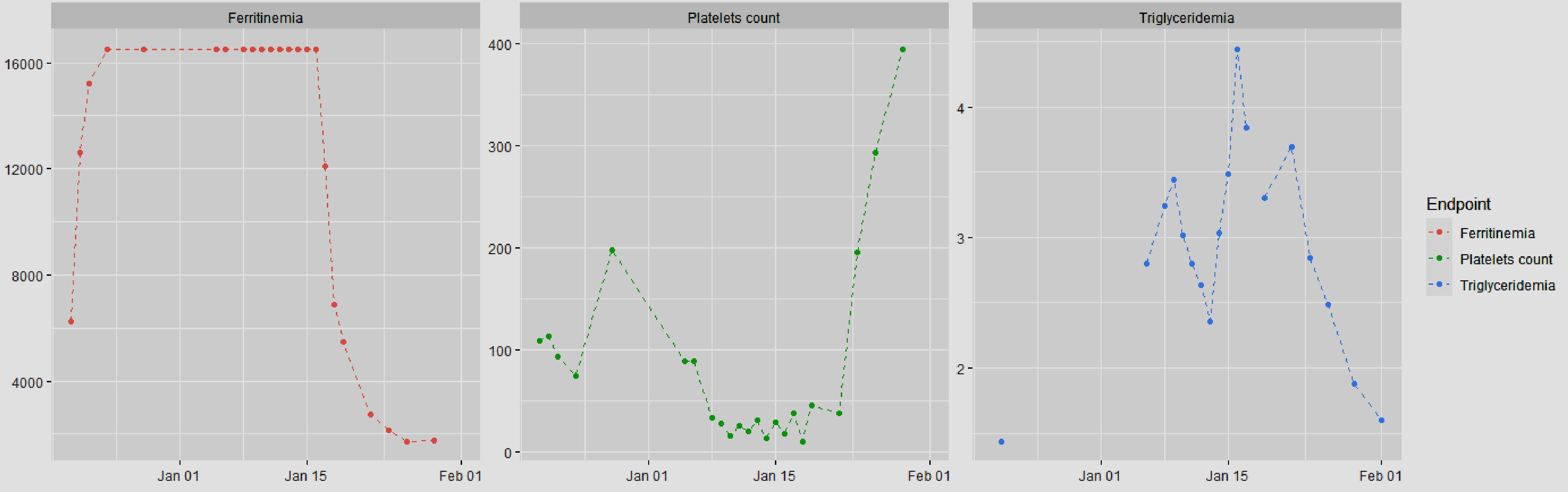 Click for large image | Figure 4. Evolution of biological markers after the introduction of ruxolitinib and corticosteroid therapy (ruxolitinib was introduced on January 13, 2024, and corticosteroid therapy on January 14, 2024). |
Diagnosis
After excluding infectious, paraneoplastic, and autoimmune causes, the primary etiology was identified as iatrogenic HLH secondary to dostarlimab immunotherapy.
Treatment
Antibiotic therapy was continued until samples tested negative. The patient received a 10-day course of tazocillin 4 g three times a day, as well as levofloxacin 750 mg daily for 8 days. Oxygen therapy was continued until rapid clinical improvement was observed after the introduction of anti-inflammatory treatment.
Following a multidisciplinary consultation with infectiologists, internists, and oncologists, a treatment plan with ruxolitinib 20 mg twice daily and methylprednisolone 80 mg daily was established, improving the patient’s condition.
Follow-up and outcomes
Ruxolitinib was hematologically tolerated, with gradual improvement in cytopenia and systemic inflammatory response parameters. Corticosteroid therapy was tapered. Other biological assessments showed a stabilized triglyceride level, a decrease in the ferritin level to 2,731 ng/mL, and a decrease in the CRP level to 29 mg/L. The complete blood count also improved (Fig. 4). Gradual tapering of corticosteroids and ruxolitinib was subsequently implemented because of the patient’s favorable clinical outcome.
Oncological management decided upon after multidisciplinary consultation consisted solely of chemotherapy, with the decision not to resume immunotherapy due to the patient experiencing grade 4 toxicity according to the Common Terminology Criteria for Adverse Events classification with a life-threatening prognosis [8]. After three cycles of chemotherapy alone, our patient showed a partial response in the primary site and a complete response in the other tumor locations.
| Discussion | ▴Top |
HLH under immunotherapy is rarely observed. Diagnosing HLH is complex, typically achieved by excluding other potential diagnoses following the HLH-94 protocol [3]. The pathophysiology of HLH remains poorly understood [2]. This condition is not widely known among medical practitioners, and its clinical signs are nonspecific, which explains the diagnostic challenges encountered by our patients. The initial symptoms in our case were severe fatigue and a febrile skin rash, which, in retrospect, were suggestive of HLH, considering the cytopenia and elevated ferritin levels. A case of HLH induced by nivolumab was reported with the initial symptom of a skin rash, but in the context of Kaposi syndrome, an HHV8-related neoplastic skin disease [9].
Once the syndromic diagnosis was established, our primary etiological hypothesis initially leaned toward pneumonia. However, this hypothesis was ruled out because of the absence of suggestive imaging findings, negative microbiological samples, and no improvement with antibiotic treatment. The possibility of EBV infection was quickly dismissed owing to the low viral load upon testing. A paraneoplastic origin was excluded based on the stability of the PET scan, and an autoimmune etiology was ruled out after an inconclusive autoimmune workup. Consequently, our focus shifted toward the iatrogenic origin of dostarlimab. The most recent treatments administered to the patient included chemotherapy and immunotherapy. Although cases of HLH involving chemotherapy have not yet been described, those involving ICI treatment have been reported. In our patient, the symptoms appeared 14 days after dostarlimab treatment. A review of clinical cases of HLH treated with ICIs showed an onset varying from days to a year, consistent with the 14-day interval post-dostarlimab injection [8]. Additionally, symptoms can appear after one or more injections, further complicating the diagnostic process. Another supporting factor for the iatrogenic etiological hypothesis is the favorable clinical and biochemical responses to the administered anti-inflammatory treatment.
Our treatment involved the administration of ruxolitinib and corticosteroids, leading to a favorable outcome characterized by rapid oxygen weaning, reduced asthenia, and biological marker improvement. For life-threatening HLH, the HLH-94 protocol, comprising treatment with corticosteroids and etoposide in an induction phase followed by hematopoietic stem cell transplantation, if necessary, is standard. The HLH-2004 protocol assessed adding cyclophosphamide in the initial phase, although this approach was not adopted because the patient was stable [10]. Dysregulation of this pathway has been implicated in the cytokine activation cascade associated with systemic inflammatory response syndrome (SIRS). Currently, ruxolitinib is used only in SIRS cases that are refractory to initial treatment; however, ongoing studies aim to introduce it earlier [11]. A literature review of 18 studies with 115 patients treated with ruxolitinib showed favorable outcomes in fever and respiratory or renal failure within 24 - 48 h [12]. We regularly monitored liver function and blood cell counts because of the risk of cytopenia. Our patient responded well to treatment, improving clinically and resuming chemotherapy. In the HLH-94 protocol, dexamethasone served as the corticosteroid. However, we administered intravenous methylprednisolone at 1 mg/kg because we were more accustomed to using this molecule than dexamethasone; dexamethasone is five to six times more potent than methylprednisolone. Tocilizumab, an interleukin-6 receptor inhibitor, is also used for quick symptom relief and corticosteroid tapering. The use of intravenous immunoglobulins and anakinra (anti-interleukin-1 agent) has also been discussed in cases of persistent organ failure [5]. The tapering of corticosteroids and ruxolitinib did not follow standardized protocols. Our collective decision was to initially taper corticosteroids over 7 - 10 days, followed by a ruxolitinib taper starting at 10 mg twice daily and then 5 mg twice daily.
Despite good clinical progress, our patient presented with several poor prognostic factors. One article described a cohort of 162 patients with HLH, identifying age, thrombocytopenia, and the presence of underlying lymphoma as the main adverse prognostic factor [13].
The patient qualified for immunotherapy due to the high MSI of her high-grade serous endometrial carcinoma. However, following serious adverse effects and a multidisciplinary discussion, the reintroduction of immunotherapy was ruled out. This decision was driven by disease progression, revealed by PET-CT re-evaluations, and the urgency to start systemic treatment. Moreover, current corticosteroid therapy exceeding 10 mg/day precludes resuming immunotherapy [14]. Furthermore, the benefit-risk ratio for rechallenge was unfavorable for our patient. However, several clinical cases have been described with a rechallenge of immunotherapy without symptom recurrence. In fact, through a literature review, Rajapakse et al [15] reported that three patients received immunotherapy without experiencing new adverse effects. If the resumption of dostarlimab in the first-line metastatic setting for endometrial carcinoma seems compromised, the question arises whether the introduction of immunotherapy is indicated in the second-line setting.
Learning points
We describe a rare case of HLH secondary to immunotherapy, notably one of the first involving dostarlimab treatment. While immunotherapy is fundamental to cancer treatment, it may cause fatal side effects. HLH management remains unstandardized due to its rarity, necessitating the exclusion of other diagnoses to confirm its iatrogenic nature. Because of the overlap of HLH symptoms with those of other clinical conditions, its diagnosis could be delayed. Our patient responded well to ruxolitinib and corticosteroids, with other anti-inflammatory treatments under investigation. Furthermore, whether immunotherapy should be restarted must be considered on a case-by-case basis.
Acknowledgments
None to declare.
Financial Disclosure
This article did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from our patient.
Author Contributions
Emilie Rollet was involved in bibliographic research and drafting and proofreading of the article. Sebastien Puigrenier, Abeer Najem, and Benoit Desgrousilliers were involved in bibliographic research and proofreading of the article. Pierre Riviere suggested this clinical case. He was involved in bibliographic research and drafting, proofreading, and submission of the article. All authors attest they meet the ICMJE criteria for authorship.
Data Availability
The data supporting the findings of this study are available from the corresponding author upon reasonable request.
Abbreviations
CMV: cytomegalovirus; CRP: C-reactive protein; EBV: Epstein-Barr virus; HLH: hemophagocytic lymphohistiocytosis; ICI: immune checkpoint inhibitor; PCR: polymerase chain reaction; RSV: respiratory syncytial virus; SIRS: systemic inflammatory response syndrome
| References | ▴Top |
- Mirza MR, Chase DM, Slomovitz BM, dePont Christensen R, Novak Z, Black D, Gilbert L, et al. Dostarlimab for primary advanced or recurrent endometrial cancer. N Engl J Med. 2023;388(23):2145-2158.
doi pubmed - Griffin G, Shenoi S, Hughes GC. Hemophagocytic lymphohistiocytosis: An update on pathogenesis, diagnosis, and therapy. Best Pract Res Clin Rheumatol. 2020;34(4):101515.
doi pubmed - Trottestam H, Horne A, Arico M, Egeler RM, Filipovich AH, Gadner H, Imashuku S, et al. Chemoimmunotherapy for hemophagocytic lymphohistiocytosis: long-term results of the HLH-94 treatment protocol. Blood. 2011;118(17):4577-4584.
doi pubmed pmc - Parikh SA, Kapoor P, Letendre L, Kumar S, Wolanskyj AP. Prognostic factors and outcomes of adults with hemophagocytic lymphohistiocytosis. Mayo Clin Proc. 2014;89(4):484-492.
doi pubmed - Shakoory B, Geerlinks A, Wilejto M, Kernan K, Hines M, Romano M, Piskin D, et al. The 2022 EULAR/ACR points to consider at the early stages of diagnosis and management of suspected haemophagocytic lymphohistiocytosis/macrophage activation syndrome (HLH/MAS). Arthritis Rheumatol. 2023;75(10):1714-1732.
doi pubmed pmc - Noseda R, Bertoli R, Muller L, Ceschi A. Haemophagocytic lymphohistiocytosis in patients treated with immune checkpoint inhibitors: analysis of WHO global database of individual case safety reports. J Immunother Cancer. 2019;7(1):117.
doi pubmed pmc - Dilies AC, Diaz L, Lesouder C, Guilpain P, Faillie JL, Palassin P, Maria A. Lymphohistiocytose hemophagocytaire associee aux inhibiteurs du point de controle immunologique: etude de pharmacovigilance. La Revue de Medecine Interne. 2021;42(Suppl1):A42-A43.
- Cancer Therapy Evaluation Program (CTEP). Accessed on March 28, 2024. Available from: https://ctep.cancer.gov/.
- Choi S, Zhou M, Bahrani E, Martin BA, Ganjoo KN, Zaba LC. Rare and fatal complication of immune checkpoint inhibition: a case report of haemophagocytic lymphohistiocytosis with severe lichenoid dermatitis. Br J Haematol. 2021;193(6):e44-e47.
doi pubmed - Bergsten E, Horne A, Arico M, Astigarraga I, Egeler RM, Filipovich AH, Ishii E, et al. Confirmed efficacy of etoposide and dexamethasone in HLH treatment: long-term results of the cooperative HLH-2004 study. Blood. 2017;130(25):2728-2738.
doi pubmed pmc - Wang J, Wang Y, Wu L, Wang X, Jin Z, Gao Z, Wang Z. Ruxolitinib for refractory/relapsed hemophagocytic lymphohistiocytosis. Haematologica. 2020;105(5):e210-e212.
doi pubmed pmc - Keenan C, Nichols KE, Albeituni S. Use of the JAK inhibitor ruxolitinib in the treatment of hemophagocytic lymphohistiocytosis. Front Immunol. 2021;12:614704.
doi pubmed pmc - Arca M, Fardet L, Galicier L, Riviere S, Marzac C, Aumont C, Lambotte O, et al. Prognostic factors of early death in a cohort of 162 adult haemophagocytic syndrome: impact of triggering disease and early treatment with etoposide. Br J Haematol. 2015;168(1):63-68.
doi pubmed - Jove M, Vilarino N, Nadal E. Impact of baseline steroids on efficacy of programmed cell death-1 (PD-1) and programmed death-ligand 1 (PD-L1) blockade in patients with advanced non-small cell lung cancer. Transl Lung Cancer Res. 2019;8(Suppl 4):S364-S368.
doi pubmed pmc - Rajapakse P, Andanamala H. Hemophagocytic lymphohistiocytosis secondary to immune checkpoint inhibitor therapy. World J Oncol. 2022;13(2):49-52.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


