| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website http://www.wjon.org |
Original Article
Volume 2, Number 5, October 2011, pages 252-258
A Pilot Study of Chemoradiotherapy With Weekly Docetaxel for Thoracic Esophageal Carcinoma With T4 and/or M1 Lymph Node Metastasis
Isamu Makinoa, c, Itasu Ninomiyaa, Koichi Okamotoa, Jun Kinoshitaa, Hironori Hayashia, Keishi Nakamuraa, Katsunobu Oyamaa, Hisatoshi Nakagawaraa, Hideto Fujitaa, Hidehiro Tajimaa, Hiroyuki Takamuraa, Hirohisa Kitagawaa, Sachio Fushidaa, Takashi Tania, Takashi Fujimuraa, Tetsuo Ohtaa, Tsuyoshi Takanakab
aDepartment of Gastroenterologic Surgery, Graduate School of Medical Science, Kanazawa University, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan
bDepartment of Radiology, Graduate School of Medical Science, Kanazawa University, 13-1 Takaramachi, Kanazawa, Ishikawa 920-8641, Japan
cCorresponding author: Isamu Makino, Email:
Manuscript accepted for publication October 14, 2011
Short title: Docetaxel for Esophageal Carcinoma
doi: https://doi.org/10.4021/wjon407w
| Abstract | ▴Top |
Background: Patients with unresectable or inoperable esophageal carcinoma are usually treated with definitive chemoradiotherapy. The present standard regimen is radiation with concurrent chemotherapy with cisplatin and fluorouracil. However, significant toxicities have been observed. The efficacy and safety of concurrent chemoradiotherapy with weekly docetaxel for head-and-neck squamous cell carcinoma and non-small cell lung cancer have already been recognized. We conducted a pilot study of definitive chemoradiotherapy with weekly docetaxel for advanced esophageal carcinoma.
Methods: Nine patients with advanced thoracic esophageal squamous cell carcinoma having a T4 tumor and/or distant lymph node metastasis (M1 LYM) were enrolled. Docetaxel was administered concurrently with 60 Gy of radiation by drip infusion at a dose of 10 mg/m2 for an hour once per week and 6 times in total.
Results: All 9 patients completed the treatment schedule without any suspension. Grade 3 or higher hematological and biochemical toxicities did not occur. Two patients achieved complete response, and 4 achieved partial response. The response rate was 67%. The median survival time was 16.2 months and the 2-year survival rate was 38.9%.
Conclusions: Concurrent chemoradiotherapy with weekly low dose docetaxel is a safe and effective treatment regimen for esophageal squamous cell carcinoma. We expect that this protocol of chemoradiotherapy may be one of the choices of treatment substituting the regimen with cisplatin and fluorouracil, particularly for the patients for whom chemotherapy with cisplatin and fluorouracil is considered inappropriate because of concomitant renal dysfunction or prior failure of systemic chemotherapy with cisplatin and fluorouracil.
Keywords: Esophageal cancer; Squamous cell carcinoma; Docetaxel; Chemoradiotherapy
| Introduction | ▴Top |
Carcinoma of the esophagus is a highly malignant disease and the treatment outcome has been poor. Patients with unresectable or inoperable disease are usually treated with definitive chemoradiotherapy. Based on the results of the Radiation Therapy Oncology Group (RTOG) phase III intergroup trial RTOG 85-01, the present standard regimen is radiation with concurrent chemotherapy with cisplatin and fluorouracil [1, 2]. In Japan, 2 phase II studies of definitive chemoradiotherapy with cisplatin and fluorouracil for advanced thoracic esophageal squamous cell carcinoma (TESCC) with T4 tumor and/or distant lymph node metastasis (M1 LYM) were performed [3, 4]. The complete response (CR) rate of each study was 33% [3] and 15% [4], and the survival rate was 23% at 3 years [3], and 31.5% at 2 years [4], respectively. However, significant toxicities, particularly severe hematological toxicities associated with these treatment regimens, have been observed [3, 4].
The efficacy and safety of concurrent chemoradiotherapy with weekly docetaxel for head-and-neck squamous cell carcinoma (HNSCC) [5-8] and non-small cell lung cancer (NSCLC) [9-12] have already been recognized. According to Japanese reports [7], the recommended dose of docetaxel in the concurrent chemoradiotherapy for HNSCC was weekly administration of 10 mg/m2.
We conducted a pilot study of concurrent chemoradiotherapy with weekly docetaxel for TESCC with T4 and/or M1 LYM. We administered docetaxel according to the protocol described in Japanese reports of HNSCC [7]. Herein, we report the efficacy and safety of this regimen.
| Patients and Methods | ▴Top |
Patients
From October 2005 to March 2009, 9 patients with advanced TESCC having a T4 tumor and/or M1 LYM were enrolled in this study. The criteria for inclusion in this study were as follows: newly diagnosed and pathologically confirmed squamous cell carcinoma of the thoracic esophagus; T4 tumor and/or M1 LYM according to the TNM classification of the UICC International Union Against Cancer, 6th edition; no evidence of esophagotracheal or esophagobronchial fistula and distant organ metastasis; performance status (PS) of 0-2 based on the classification criteria of the Eastern Cooperative Oncology Group; age 20-80 years; adequate bone marrow [white blood cell (WBC) count ≥ 3,000 /µl, neutrocyte count ≥ 1,500 /µl, hemoglobin (Hb) level ≥ 10.0 g/dl, platelet count ≥ 100,000 /µl], hepatic (total bilirubin < 1.5 mg/dl, alanine transaminase (ALT), aspartate transaminase (AST) < 3.0 × normal limit), pulmonary (PO2 ≥ 60 mmHg), and renal (serum creatinine < 2.0 mg/dl, creatinine clearance ≥ 50 ml/min) functions. Patients with other active synchronous carcinoma, concurrent uncontrolled medical illness, and prior chemotherapy and radiotherapy for any neoplasm were excluded. All patients provided written informed consent before enrollment in this study.
Treatment schedule
The treatment schedule is summarized in Fig. 1. Docetaxel was administered concurrently with radiotherapy by drip infusion at a dose of 10 mg/m2 for an hour once per week and 6 times in total. Radiation was administered via 10 MV X-ray (1 fraction per day and 5 fractions per week). When the tumor was located in the upper or middle third of the thoracic esophagus, the treatment volume included bilateral supraclavicular lymph nodes as well as the mediastinum in a T-shaped pattern. When the tumor was located in the lower third of the esophagus, the mediastinum and celiac axis lymph nodes were irradiated and the supraclavicular lymph nodes were excluded if the cervical nodes tested negative. Oblique fields were used to spare the spinal cord after 40 Gy of radiation was delivered by antero-posterior opposed pair portals. Weekly administration of docetaxel was skipped when the WBC, neutrocyte, or platelet count decreased to < 3,000 /µl, < 1,500 /µl, or < 75,000 /µl, respectively. Radiotherapy was suspended when the WBC, neutrocyte, or platelet count decreased to < 1,000 /µl, < 500 /µl, or < 50,000 /µl, and was resumed when the counts recovered to ≥ 1,000 /µl, ≥ 500 /µl, and ≥ 50,000 /µl, respectively.
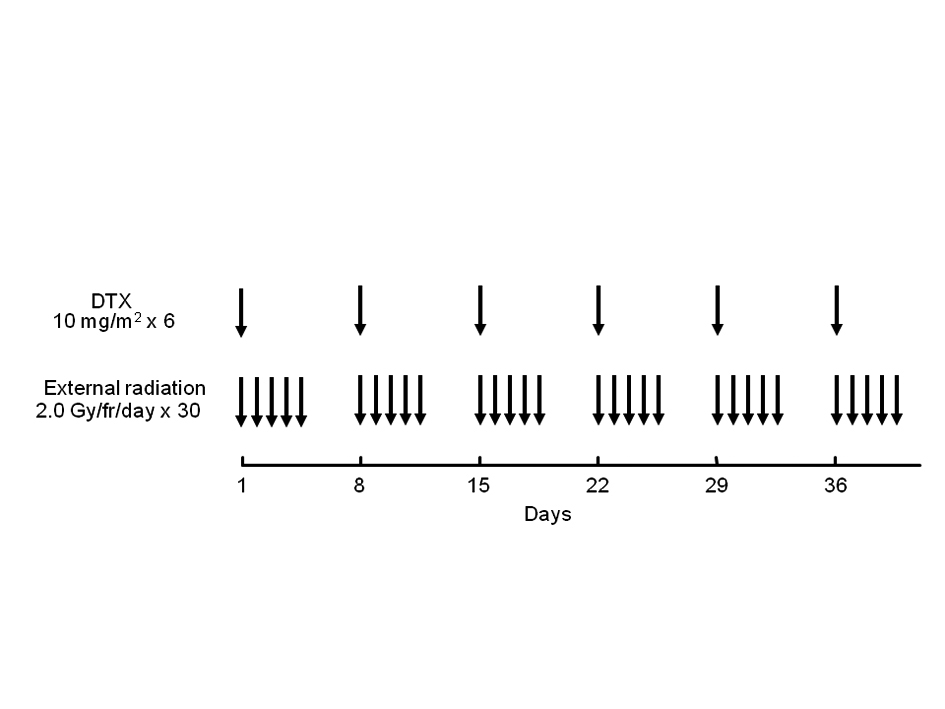 Click for large image | Figure 1. Treatment schedule of chemoradiotherapy with weekly docetaxel. DTX: docetaxel. |
Evaluation of toxicity and response
Adverse reactions were evaluated according to the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE), version 4.0. The tumor response associated with the treatment was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) [13]. The response in primary tumors, which were considered as non-target lesions, was examined by endoscopy and evaluated according to the Response Evaluation Criteria for Primary Lesion using Endoscopy defined in the Japanese Classification of Esophageal Cancer published by the Japanese Esophageal Society [14].
Statistical analysis
Overall survival time was defined as the time from initiation of the treatment to the date of death or date of final follow-up examination. Survival rates were estimated by the Kaplan-Meier method. All statistical calculations were carried out using SPSS software version 11 (SPSS Inc; Chicago, IL).
| Results | ▴Top |
Patient characteristics
Between October 2005 and March 2009, 9 patients were enrolled in this study. The characteristics of these 9 patients are listed in Table 1. The median age was 66 years (range, 51 to 78 years). There were 4 patients with non-T4 M1 LYM, 4 patients with T4 M0, and 1 patient with T4 M1 LYM. Four patients had a T4 tumor that invaded into the tracheobronchial tree and 1 patient had a T4 tumor that invaded into both the tracheobronchial tree and thoracic aorta. Among the 5 patients with M1 LYM, 4 patients had cervical lymph node metastases, and 1 patient had both cervical and abdominal lymph node metastases.
 Click to view | Table 1. Patient characteristics |
Toxicity
All patients completed treatment with a total radiation dose of 60 Gy without any suspension. The most severe toxicities during the treatment and follow-up period are summarized in Table 2. Grade 3 or higher hematological and biochemical toxicities did not occur. The most frequent toxicities were anorexia and esophagitis. A patient with T4 disease developed treatment-related perforation of the esophageal wall and died because of esophago-mediastinal fistula 27 months after initiation of the treatment. There was 1 (11%) death related to the treatment.
 Click to view | Table 2. Summary of toxicity |
Response
Individual data of the treatment response and prognosis are listed in Table 3. Of the 9 patients, 2 (22%) achieved CR, and 4 (44%) achieved PR. The response rate was 67% (Table 4). The other 3 patients (33%) had progressive disease (PD). PD in these 3 patients developed because of progression of the primary lesion, development of lung metastases, and development of bone metastases, respectively. As far as the response of primary tumors were concerned, 4 patients (44%), including one with T4 disease, achieved CR.
 Click to view | Table 3. Treatment response and prognosis of 9 patients |
 Click to view | Table 4. Summary of response and survival |
Survival
The prognoses of the 9 patients are listed in Table 3, and the overall survival curves are shown in Fig. 2. The median survival time (MST) was 16.2 months (95% confidence interval: 4.9-27.5 months) and the 2-year survival rate was 38.9% (Table 4).
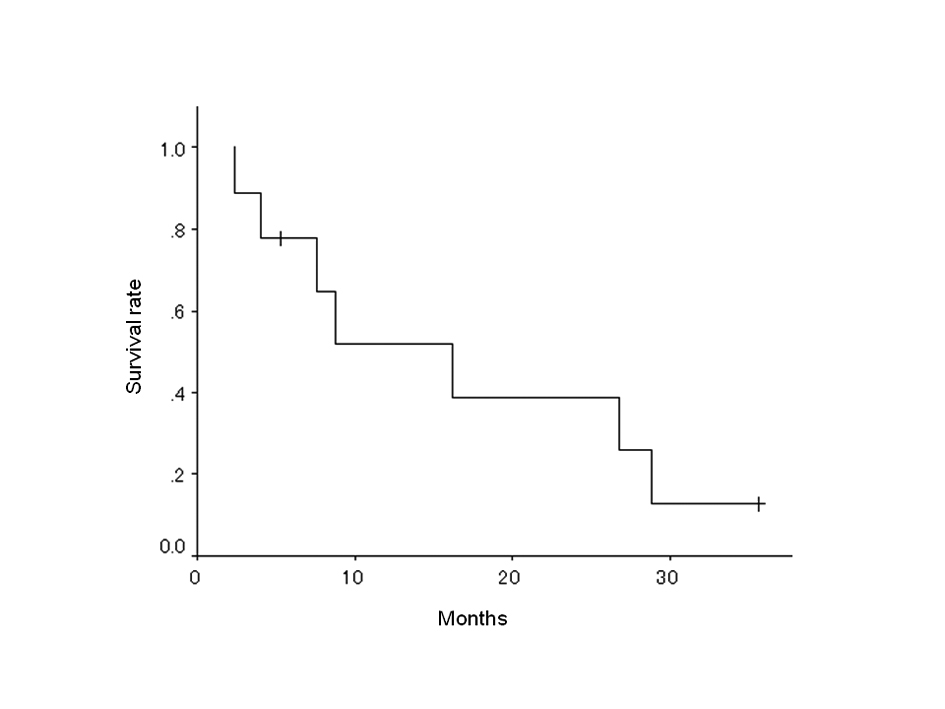 Click for large image | Figure 2. Overall survival of 9 patients. |
| Discussion | ▴Top |
The present standard regimen of chemoradiotherapy for TESCC is radiation and concurrent chemotherapy with cisplatin and fluorouracil [1-4]. However, because other treatment regimens have not been established, there is no recommended choice of treatment for patients for whom chemotherapy with cisplatin and fluorouracil is considered inappropriate because of concomitant renal dysfunction or prior failure of systemic chemotherapy with cisplatin and fluorouracil. Moreover, significant toxicities, particularly severe hematological toxicities, associated with this treatment regimen, have been observed [1-4]. Other effective and reliable chemoradiotherapy regimens in addition to the one with cisplatin and fluorouracil are needed.
The concurrent use of taxanes (docetaxel and paclitaxel) with radiation has been employed for the treatment of patients with HNSCC [5-8] and NSCLC [9-12]. Many clinical and experimental studies have demonstrated the efficacy of concomitant administration of taxanes with radiation [15-22]. The efficacy of taxanes is based on not only their cytotoxicity but also their radio-sensitizing effect. Microtubule modification initiated by taxanes causes cells to accumulate in the radio-sensitive G2/M phase of the cell cycle. The radio-sensitizing effect of taxanes is thought to be derived mainly from this mechanism. Furthermore, in vitro and in vivo studies have demonstrated roles for the p53 pathways and apoptotic mechanism as contributing factors of the therapeutic benefits of combining taxanes with concurrent radiation.
Taxanes are usually administered every 3 weeks as a single cytotoxic agent; however, there are several reports showing that weekly administration of taxanes may be the most effective dosing regimen in combination with radiation [12, 23]. Most of regimens of docetaxel with concurrent radiotherapy consist of weekly administration of docetaxel at a dose of 10-30 mg/m2 [5-12]. The most appropriate dosage of docetaxel may be different among the types of tumor, range of irradiation fields, and ethnic origin of the patients. In this pilot study, we administered docetaxel according to the protocol described in Japanese reports of HNSCC [7], considering the racial identity and oncological similarity between TESCC and HNSCC.
In the present study, the overall CR and response rate were 22% and 67%, respectively. The median survival time was 16.2 months and the 2-year survival rate was 38.9%. These results are compatible to those in previous Japanese reports of concurrent chemoradiotherapy with cisplatin and fluorouracil for TESCC with T4 and/or M1 LYM [3, 4]. However, grade 3 or higher hematological toxicities did not occur in the present study, although these complications occurred in approximately 20%-30% of patients in the previous Japanese reports [3, 4]. In our study, all 9 patients completed the treatment schedule without any suspension. A critical adverse event occurred in 1 of the 9 patients (11%), who developed perforation of the esophageal wall and died because of esophago-mediastinal fistula. Although the present study has a limited number of patients, concurrent chemoradiotherapy with weekly low dose docetaxel might be a feasible and promising treatment regimen for TESCC.
We expect that this protocol of chemoradiotherapy may be one of the choices of treatment for TESCC, particularly for the patients for whom chemotherapy with cisplatin and fluorouracil is considered inappropriate because of concomitant renal dysfunction or prior failure of systemic chemotherapy with cisplatin and fluorouracil. Moreover, as one of the treatment options, we plan to combine this regimen with prior induction chemotherapy with cisplatin and fluorouracil to achieve a survival benefit for the patients with advanced TESCC. We are currently conducting a phase I/II study of chemoradiotherapy with docetaxel following induction chemotherapy with cisplatin and fluorouracil in order to determine the appropriate dosage for the weekly administration of docetaxel.
| References | ▴Top |
- Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA
Jr , Al-Sarraf M, Byhardt R,et al . Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiation Therapy Oncology Group. JAMA. 1999;281(17):1623-1627.
pubmed doi - Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, Okawara G,
et al . INT 0123 (Radiation Therapy Oncology Group 94-05) phase III trial of combined-modality therapy for esophageal cancer: high-dose versus standard-dose radiation therapy. J Clin Oncol. 2002;20(5):1167-1174.
pubmed doi - Ohtsu A, Boku N, Muro K, Chin K, Muto M, Yoshida S, Satake M,
et al . Definitive chemoradiotherapy for T4 and/or M1 lymph node squamous cell carcinoma of the esophagus. J Clin Oncol. 1999;17(9):2915-2921.
pubmed - Ishida K, Ando N, Yamamoto S, Ide H, Shinoda M. Phase II study of cisplatin and 5-fluorouracil with concurrent radiotherapy in advanced squamous cell carcinoma of the esophagus: a Japan Esophageal Oncology Group (JEOG)/Japan Clinical Oncology Group trial (JCOG9516). Jpn J Clin Oncol. 2004;34(10):615-619.
pubmed doi - Tishler RB, Norris CM
Jr , Colevas AD, Lamb CC, Karp D, Busse PM, Nixon A,et al . A Phase I/II trial of concurrent docetaxel and radiation after induction chemotherapy in patients with poor prognosis squamous cell carcinoma of the head and neck. Cancer. 2002;95(7):1472-1481.
pubmed doi - Tishler RB, Posner MR, Norris CM
Jr , Mahadevan A, Sullivan C, Goguen L, Wirth LJ,et al . Concurrent weekly docetaxel and concomitant boost radiation therapy in the treatment of locally advanced squamous cell cancer of the head and neck. Int J Radiat Oncol Biol Phys. 2006;65(4):1036-1044.
pubmed doi - Fujii M, Tsukuda M, Satake B, Kubota A, Kida A, Kohno N, Okami K,
et al . Phase I/II trial of weekly docetaxel and concomitant radiotherapy for squamous cell carcinoma of the head and neck. Int J Clin Oncol. 2004;9(2):107-112.
pubmed doi - Fukada J, Shigematsu N, Takeda A, Ohashi T, Tomita T, Shiotani A, Kunieda E,
et al . Weekly low-dose docetaxel-based chemoradiotherapy for locally advanced oropharyngeal or hypopharyngeal carcinoma: a retrospective, single-institution study. Int J Radiat Oncol Biol Phys. 2010;76(2):417-424.
pubmed doi - Hainsworth JD, Vokes EE. Docetaxel (Taxotere) in combination with radiation therapy and the potential of weekly administration in elderly and/or poor performance status patients with advanced non-small cell lung cancer. Semin Oncol. 2001;28(1 Suppl 2):22-27.
pubmed doi - Onishi H, Kuriyama K, Yamaguchi M, Komiyama T, Tanaka S, Araki T, Nishikawa K,
et al . Concurrent two-dimensional radiotherapy and weekly docetaxel in the treatment of stage III non-small cell lung cancer: a good local response but no good survival due to radiation pneumonitis. Lung Cancer. 2003;40(1):79-84.
pubmed doi - Brunsvig PF, Hatlevoll R, Berg R, Lauvvang G, Owre K, Wang M, Aamdal S. Weekly docetaxel with concurrent radiotherapy in locally advanced non-small cell lung cancer: a phase I/II study with 5 years' follow-up. Lung Cancer. 2005;50(1):97-105.
pubmed doi - Brunsvig PF, Andersen A, Aamdal S, Kristensen V, Olsen H. Pharmacokinetic analysis of two different docetaxel dose levels in patients with non-small cell lung cancer treated with docetaxel as monotherapy or with concurrent radiotherapy. BMC Cancer. 2007;7:197.
pubmed - Japan Esophageal Society. Japanese classifi cation of esophageal cancer 10th edition. Kanehara, Tokyo.
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J,
et al . New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92(3):205-216.
pubmed doi - Ringel I, Horwitz SB. Studies with RP 56976 (taxotere): a semisynthetic analogue of taxol. J Natl Cancer Inst. 1991;83(4):288-291.
pubmed doi - Gueritte-Voegelein F, Guenard D, Lavelle F, Le Goff MT, Mangatal L, Potier P. Relationships between the structure of taxol analogues and their antimitotic activity. J Med Chem. 1991;34(3):992-998.
pubmed doi - Tishler RB, Geard CR, Hall EJ, Schiff PB. Taxol sensitizes human astrocytoma cells to radiation. Cancer Res. 1992;52(12):3495-3497.
pubmed - Tishler RB, Schiff PB, Geard CR, Hall EJ. Taxol: a novel radiation sensitizer. Int J Radiat Oncol Biol Phys. 1992;22(3):613-617.
pubmed doi - Choy H, Rodriguez FF, Koester S, Hilsenbeck S, Von Hoff DD. Investigation of taxol as a potential radiation sensitizer. Cancer. 1993;71(11):3774-3778.
pubmed doi - Wahl AF, Donaldson KL, Fairchild C, Lee FY, Foster SA, Demers GW, Galloway DA. Loss of normal p53 function confers sensitization to Taxol by increasing G2/M arrest and apoptosis. Nat Med. 1996;2(1):72-79.
pubmed doi - Hennequin C, Giocanti N, Favaudon V. Interaction of ionizing radiation with paclitaxel (Taxol) and docetaxel (Taxotere) in HeLa and SQ20B cells. Cancer Res. 1996;56(8):1842-1850.
pubmed - Milas L, Milas MM, Mason KA. Combination of taxanes with radiation: preclinical studies. Semin Radiat Oncol. 1999;9(2 Suppl 1):12-26.
pubmed - Hainsworth JD, Burris HA
3rd , Greco FA. Weekly administration of docetaxel (Taxotere): summary of clinical data. Semin Oncol. 1999;26(3 Suppl 10):19-24.
pubmed
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


