| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 5, October 2022, pages 259-271
Distinct Prognostic Factors of Ground Glass Opacity and Pure-Solid Lesion in Pathological Stage I Invasive Lung Adenocarcinoma
Wen Yu Zhaia, d, Wing Shing Wonga, d, Fang Fang Duanb, d, Da Chuan Lianga, Li Gonga, Shu Qin Daic, e, Jun Ye Wanga, e
aDepartment of Thoracic Surgery, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
bDepartment of Medical Oncology, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
cDepartment of Laboratory Medicine, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou, Guangdong, China
dThese authors contributed equally to drafting this manuscript.
eCorresponding Author: Jun Ye Wang and Shu Qin Dai, State Key Laboratory of Oncology in South China, Collaborative Innovation Center for Cancer Medicine, Sun Yat-sen University Cancer Center, Guangzhou 510060, Guangdong, Chinaand
Manuscript submitted May 20, 2022, accepted August 1, 2022, published online October 22, 2022
Short title: GGO and Pure-Solid Patients
doi: https://doi.org/10.14740/wjon1499
| Abstract | ▴Top |
Background: Ground glass opacity (GGO) is associated with favorable survival in lung cancer. However, the relevant evidence of the difference in prognostic factors between GGO and pure-solid nodules for pathological stage I invasive adenocarcinoma (IAC) is limited. We aimed to identify the impact of GGO on survival and find prognostic factor for part-GGO and pure-solid patients.
Methods: Between December 2007 and August 2018, patients with pathological stage I IAC were retrospectively reviewed and categorized into the pure-GGO, part-GGO, and pure-solid groups. Survival curves were analyzed by the Kaplan-Meier method and compared by log-rank tests. Least absolute shrinkage and selection operator and Cox regression models were used to obtained prognostic factors for disease-free survival (DFS) and overall survival (OS).
Results: The number of patients with pure-GGO, part-GGO, and pure-solid was 134, 540, and 396, respectively. Part-GGO patients with consolidation-tumor-ratio (CTR) > 0.75 had similar outcome to those with pure-solid nodules. In part-GGO patients, CTR was negatively associated with OS (P = 0.007) and solid tumor size (STS) was negatively associated with DFS (P < 0.001). Visceral pleural invasion (VPI) was negatively associated with OS (P = 0.040) and DFS (P = 0.002). Sublobectomy was negatively associated with OS (P = 0.008) and DFS (P = 0.005), while extended N1 stations examination was associated with improved DFS (P = 0.005) in pure-solid patients.
Conclusions: Though GGO component is a positively prognostic factors of patients with pathological stage I IAC, a small proportion of GGO components is not associated with favorable survival. VPI, STS and CTR are the significant predictors for part-GGO patients. Sublobectomy, especially wedge resection should be used cautiously in pure-solid patients.
Keywords: Lung adenocarcinoma; Ground glass opacity; Solid tumor size; Consolidation tumor ratio; Prognostic factor
| Introduction | ▴Top |
Ground glass opacity (GGO) is defined as a hazy opacity without obscuring the underlying pulmonary vessels or bronchial structures in the lung window [1]. Substantial studies have demonstrated that the presence of the GGO component is associated with better prognosis in patients with non-small cell lung cancer (NSCLC) [2-4]. However, there are still some questions that need further exploration.
A considerable proportion of nodules with GGO components were diagnosed as adenocarcinoma in situ (AIS) and minimally invasive adenocarcinoma (MIA), while patients with AIS or MIA were observed to have no recurrence and the 5-year overall survival (OS) rate was nearly 100% [5, 6]. At the same time, the 5-year OS rate for pathological stage I patients was only from 73% to 90% [7]. It is not difficult to conclude that almost all recurrent patients with adenocarcinoma suffered from invasive adenocarcinoma (IAC). However, evidence especially concentrated on the significance of GGO components in patients with pathological stage I IAC is limited.
There has long been discussed whether GGO containing early-stage lung cancers should be staged differently from those pure-solid ones [2, 6, 8]. Whereas the practical extension of previous studies was limited due to their neglect in different prognostic factors between GGO and pure-solid nodules, which might play vital roles in identifying patients at high risk and making individual treatment and follow-up plans. Only few studies reported the distinct prognostic factors of GGO and pure-solid nodules in stage I NSCLC, which included a large proportion of lung squamous carcinoma (LUSC). It is well known that almost all LUSCs is pure-solid nodules while lung adenocarcinoma (LUAD) and LUSC also differ greatly in prognosis and treatment. Hence, one study included only stage I IAC is needed.
The eighth staging system for cT stage is based on solid tumor size (STS) rather than whole tumor size. However, the prognostic value of STS or consolidation-tumor-ratio (CTR) is poorly understood and controversial. Su et al reported that CTR rather than STS is an independent prognostic factor for stage I LADC [9]. Nakada et al reviewed 32 publications and concluded that STS or mediastinal diameter instead of CTR are optimal prognostic radiological tools for stage I NSCLC [10]. Ye et al analyzed 841 GGOs and noted that none of CTR and STS could predict the prognosis [11].
Mutations of epidermal growth factor receptor (EGFR) and anaplastic lymphoma kinase (ALK), which play an important role in the treatment of LUAD, exist in a large proportion of LUAD [12]. It is valuable to be aware of the relation between the GGO pattern and gene mutation, but the relevant report of their association is inadequate.
Therefore, we reviewed patients with stage I IAC from a large, homogeneous cohort at our institution to identify the value of GGO components on prognosis and find differences in prognostic factor and gene mutation status between part-GGO and pure-solid patients. We also aimed to investigate whether CTR or STS was associated with prognosis of patients with part-GGO.
| Patients and Methods | ▴Top |
Patients
Patients with IA-IB LADC who received radical surgery between January 2012 and June 2018 at the Sun Yat-sen University Cancer Center were retrospectively reviewed in this study. We obtained approval from the Institutional Review Board (IRB) of the Sun Yat-sen University Cancer Center (IRB No. SZR2019-108), and the written informed consent for this retrospective study was waived. This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Key inclusion criteria were as follows: 1) pathological diagnosis of stage IA-IB (pT1a-T2aN0M0) LUAD; 2) confirmed negative surgical margin (R0). Patients who met the following criteria were excluded: 1) received neoadjuvant therapy; 2) multiple primary tumors; 3) death within 1 month after surgical resection due to any cause; 4) pathological diagnosis of AIS and MIA. The tumor pathologic staging was based on the eighth American Joint Committee on Cancer (AJCC) staging edition [13].
Radiological evaluation gene testing methods
Thin slice computed tomography (CT) scans were reviewed by two radiologists independently to measure the GGO and consolidation component. Tumor size was defined as the maximum diameter in the axial plane in lung window. STS was defined as the maximum diameter of the solid component. CTR was defined as the ratio of the STS to the tumor size. For the pure-GGO tumor, CTR = 0, for the part-GGO tumor, 0 < CTR < 1 and for the pure-solid tumor, CTR = 1 [14]. For EGFR mutations detecting, DNA extraction and analysis of exons 18, 19, 20, and 21 were performed using amplification refractory mutation system-polymerase chain reaction (ARMS-PCR). EML4-ALK fusion was screened with reverse transcriptase-polymerase chain reaction (RT-PCR) and immunohistochemistry, confirmed with fluorescent in situ hybridization (FISH).
Follow-up and endpoints
The follow-up was performed every 3 months for the first 2 years, every 6 months until 5 years and once a year thereafter; it mainly included routine blood examination and chest CT scans. Brain magnetic resonance imaging, bone scintigraphy, and positron emission tomography were performed if necessary.
The main endpoints of this study were the overall survival time (OS) and the disease-free survival time (DFS). The DFS was defined as the time from the date of the surgery to the date of the first event recurrence or death due to any cause; and the OS was calculated from the date of operation to the date of death due to any cause or the last follow-up.
Statistical analysis
Continuous data are shown as the mean ± standard deviation (SD) or median and were compared using Student’s t-test or analysis of variance test. Pearson’s χ2, Kruskal-Wallis H test or Mann-Whitney U test was used to compare categorical data. We used the Kaplan-Meier method to calculate survival curves and log-rank test to compare the survival. Cox proportional hazards regression models were used to identify prognostic factors. Factors with a P value less than 0.05 in the univariate Cox model were further entered in the least absolute shrinkage and selection operator (LASSO) analysis to remove multicollinearity. Factors with statistical significance in LASSO analysis were finally entered in the multivariate Cox regression analysis. Factors assessed in this study mainly included: sex, age, pathological tumor size, STS, visceral pleural invasion (VPI), CT characteristics, smoking history, differentiation degree, TNM stage, vascular invasion, operative approach, number of N2 station examined, number of N1 station examined, and CTR. All statistical analyses were performed using SPSS software version 22.0 for Windows (SPSS Inc, Chicago, IL, USA) and R software (version 4.0.3; http://www.r-project.org). P value < 0.05 was seen as statistical significance and the reported significance levels were all two-sided.
| Results | ▴Top |
Patient characteristics
The baseline characteristics of 1070 patients with pathological stage I IAC are shown in Table 1. The median age of the investigated 545 male and 525 female patients was 61 (range 29 to 82 years). The number of patients with pure-GGO, part-GGO, and pure-solid was 134, 540, and 396, respectively. These three groups differed in gender (P = 0.008), age (P < 0.001), solid component size (P < 0.001), smoking history (P < 0.001), eighth TNM stage (P < 0.001), differentiation degree (P < 0.001), VPI (P < 0.0001), vascular invasion (P < 0.001), number of N2 station examined (P < 0.001), surgical approach (P < 0.001), and thoracotomy or video-assisted thoracoscopic surgery (VATS) (P < 0.001) were significantly different. When only comparing patients with part-GGO and pure-solid, patients in pure-solid group had a larger solid component size (P < 0.001), more smokers (P = 0.028), more patients in stage IB (P < 0.001), more patients with poor differentiation degree (P < 0.001) and VPI (P = 0.009), and less patients received sublobectomy (P = 0.022). When excluding patients with unknown EGFR and ALK status, we observed a tendency that patients in the part GGO group had a higher rate of EGFR gene mutation (59.9% versus 53.2%, P = 0.084).
 Click to view | Table 1. Patient Characteristics |
Survival analysis in entire cohort
The median follow-up time of entire cohort was 48.2 months. The 5-year OS rate of patients in pure-GGO, part-GGO, and pure-solid group was 99.1%, 92.6%, and 86.0%, respectively (log-rank P = 0.0021) (Fig. 1a). Only one patient died after resection in the pure-GGO group and there was no apparent difference in OS between the part-GGO and pure-solid group (log-rank P = 0.051). The 5-year DFS rate of patients in the pure-GGO, part-GGO, and pure-solid group was 93.1%, 85.4%, and 72.3%, respectively (log-rank P < 0.0001), (Fig. 1b). Table 2 and Supplementary Material 1 (www.wjon.org) shows the LASSO regression and Cox proportional risk regression results for OS and DFS in the entire cohort. In the analysis of OS, advanced age (P = 0.001; hazard ratio (HR) 1.042; 95% confidence interval (CI): 1.017 - 1.067) and VPI (P = 0.009; HR 1.748; 95% CI: 1.153 - 2.652) were negatively correlated with OS. Female (P = 0.003; HR 0.509; 95% CI: 0.327 - 0.793) and pure-GGO (P = 0.032; HR 0.114; 95% CI: 0.016 - 0.834) were positively correlated with OS. In the analysis of DFS, larger pathological tumor size (P < 0.001; HR 1.451; 95% CI: 1.205 - 1.747) and VPI (P < 0.001; HR 1.698; 95% CI: 1.265 - 2.279) were the negatively prognostic factors of DFS. Female sex (P = 0.007; HR 0.663; 95% CI: 0.495 - 0.888), the number of N1 stations examined (P =0.01; HR 0.844; 95% CI: 0.742 - 0.960), and GGO component: part-GGO (P = 0.001; HR 0.605; 95% CI: 0.449 - 0.815), pure-GGO (P = 0.003; HR 0.252; 95% CI: 0.101 - 0.629) were the positively prognostic factors of DFS.
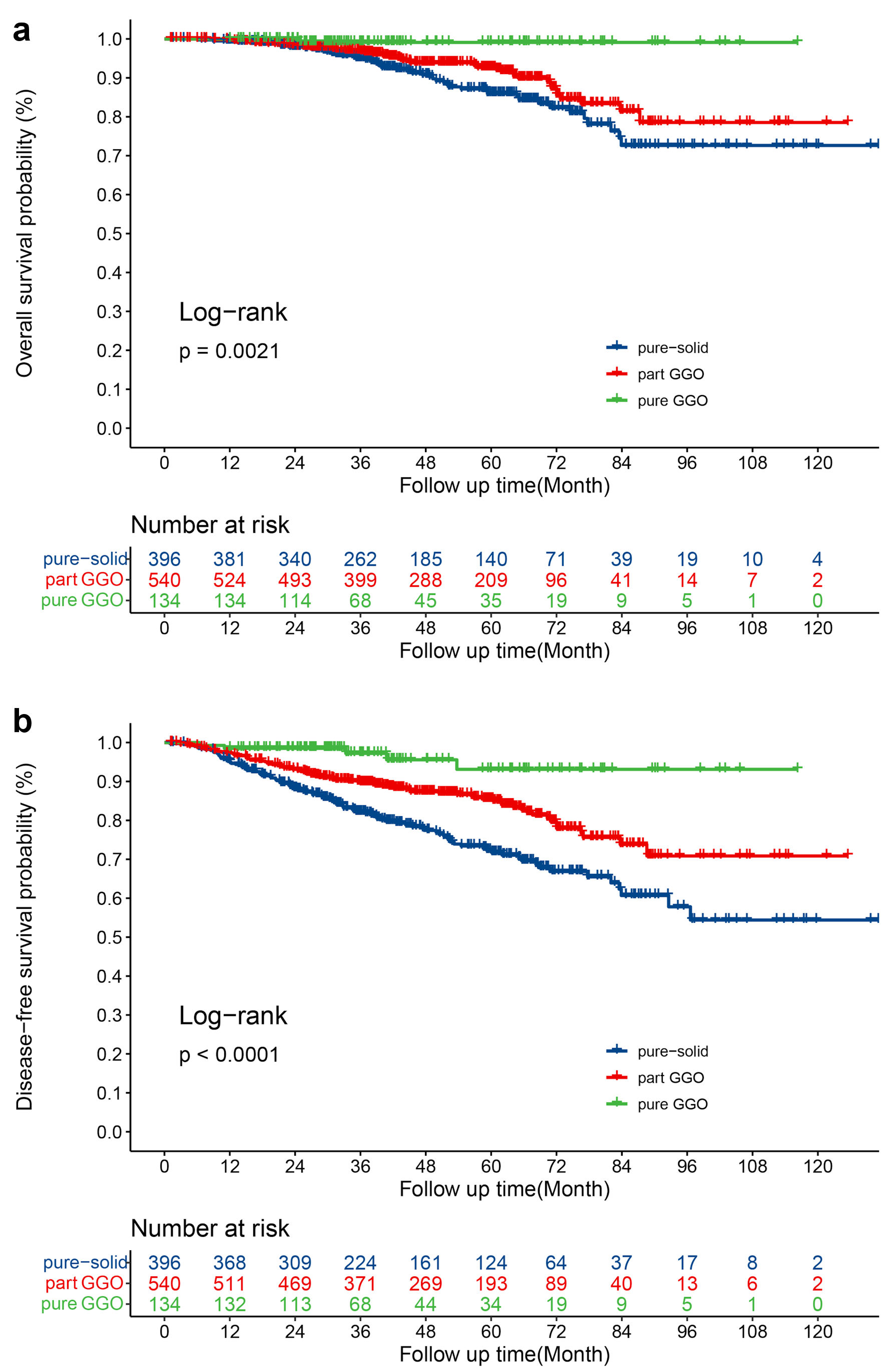 Click for large image | Figure 1. (a) OS for patients with pure-GGOs, part-GGOs and pure-solid nodules. (b) DFS for patients with pure-GGOs, part-GGOs and pure-solid nodules. OS: overall survival; DFS: disease-free survival; GGO: ground-glass opacity. |
 Click to view | Table 2. LASSO and Cox Regression Analyses for All Patients |
Survival analysis in part-GGO group
To identify prognostic factors for patients with part-GGO, we performed LASSO and Cox analysis in the part-GGO group and results are shown in Table 3 and Supplementary Material 2 (www.wjon.org). In the analysis of OS, female sex (P = 0.001; HR 0.329; 95% CI: 0.170 - 0.635) was positively associated with OS, while CTR (P = 0.007; HR 1.402; 95% CI: 1.096 - 1.792) and VPI (P = 0.040; HR 1.894; 95% CI: 1.029 - 3.485) were negatively associated with OS. In the analysis of DFS, female sex (P < 0.001; HR 0.381; 95% CI: 0.236 - 0.613) was the positively prognostic factor of DFS while STS (P < 0.001; HR 1.773; 95% CI: 1.372 - 2.290) and VPI (P = 0.002; HR 2.022; 95% CI: 1.296 - 3.154) were the negatively prognostic factors of DFS.
 Click to view | Table 3. LASSO and Cox Regression Analyses for Patients With Part-GGOs |
To further investigate the impact of CTR on survival, we divided the part-GGO group into three subgroups according to CTR: 0 <CTR ≤ 0.25, 0.25 < CTR ≤ 0.75, and 0.75 < CTR <1, and compared the survival with pure-GGO and pure-solid. As shown in Figure 2, there was a significant difference in OS (log-rank P = 0.00093) and DFS (log-rank P < 0.0001) of patients with different rank of CTR. Interestingly, there were no significant difference in OS and DFS between CTR = 0 and 0 <CTR ≤ 0.25 (OS: log-rank P = 0.639; DFS: log-rank P = 0.334) as well as 0.75 < CTR < 1 and CTR = 1 (OS: log-rank P = 0.992; DFS: log-rank P = 0.245) (Fig. 2a, b).
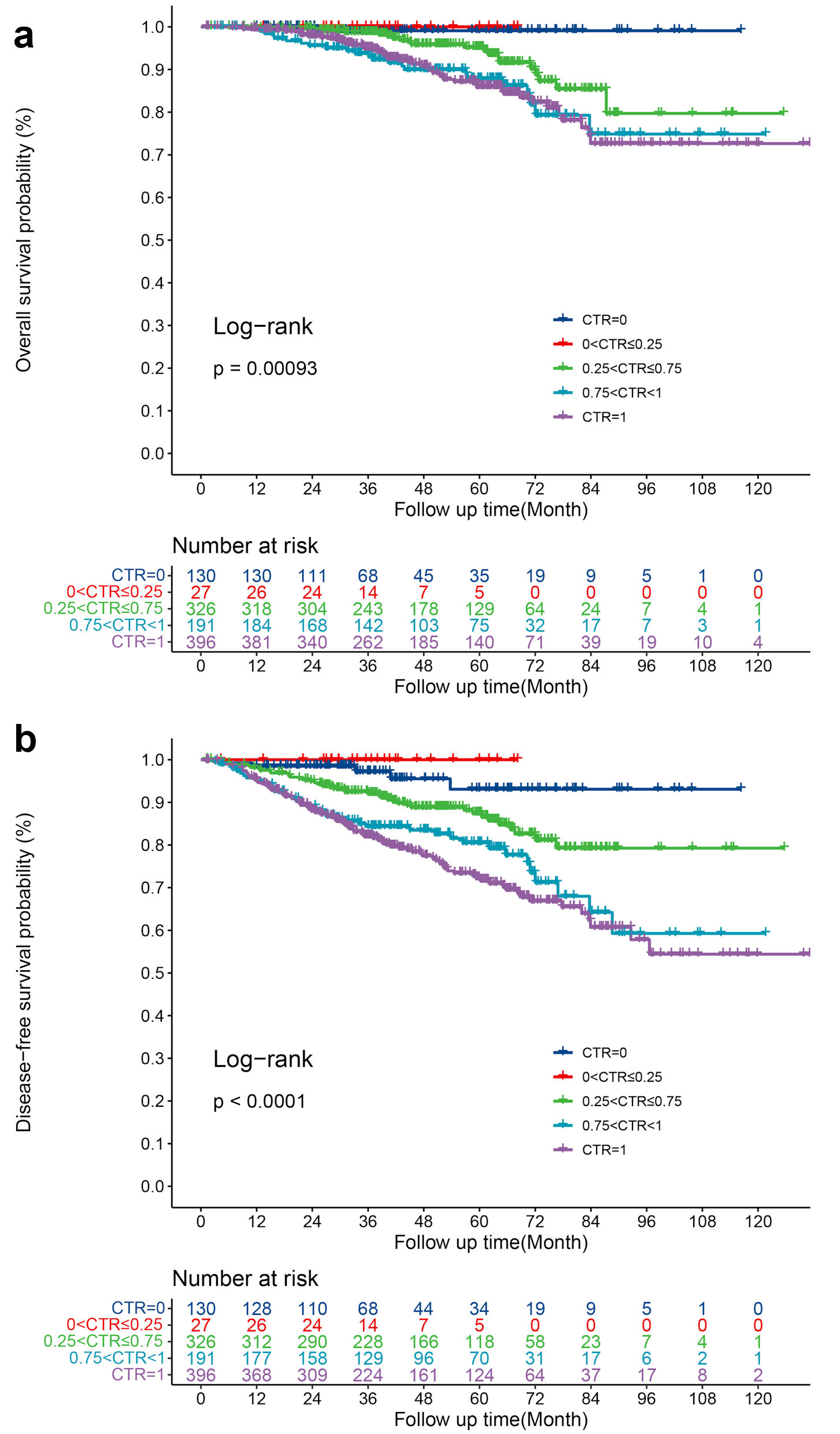 Click for large image | Figure 2. (a) OS for patients with different grade of CTR. (b) DFS for patients with different grade of CTR. OS: overall survival; DFS: disease-free survival; CTR: consolidation-tumor-ratio. |
Survival analysis in pure-solid group
In the pure-solid group, patients who received sublobectomy had a worse OS (log-rank P = 0.0010), (Fig. 3a) and DFS (log-rank P = 0.00071), (Fig. 3b). Table 4 and Supplementary Material 3 (www.wjon.org) showed the Lasso and Cox regression analyses in pure-solid group. Advanced age (P < 0.001; HR 1.073; 95% CI: 1.035 - 1.112) and sublobectomy (P = 0.008; HR 4.118; 95% CI: 1.455 - 11.657) were negative prognostic factors of OS while larger pathological tumor size (P = 0.012; HR 1.377; 95% CI: 1.074 - 1.766) and sublobectomy (P = 0.005; HR 3.150; 95% CI: 1.401 - 7.081) were negative prognostic factors for DFS. In addition, patients with extended N1 stations examined (P = 0.008; HR 0.517; 95% CI: 0.317 - 0.842) were associate with improved DFS.
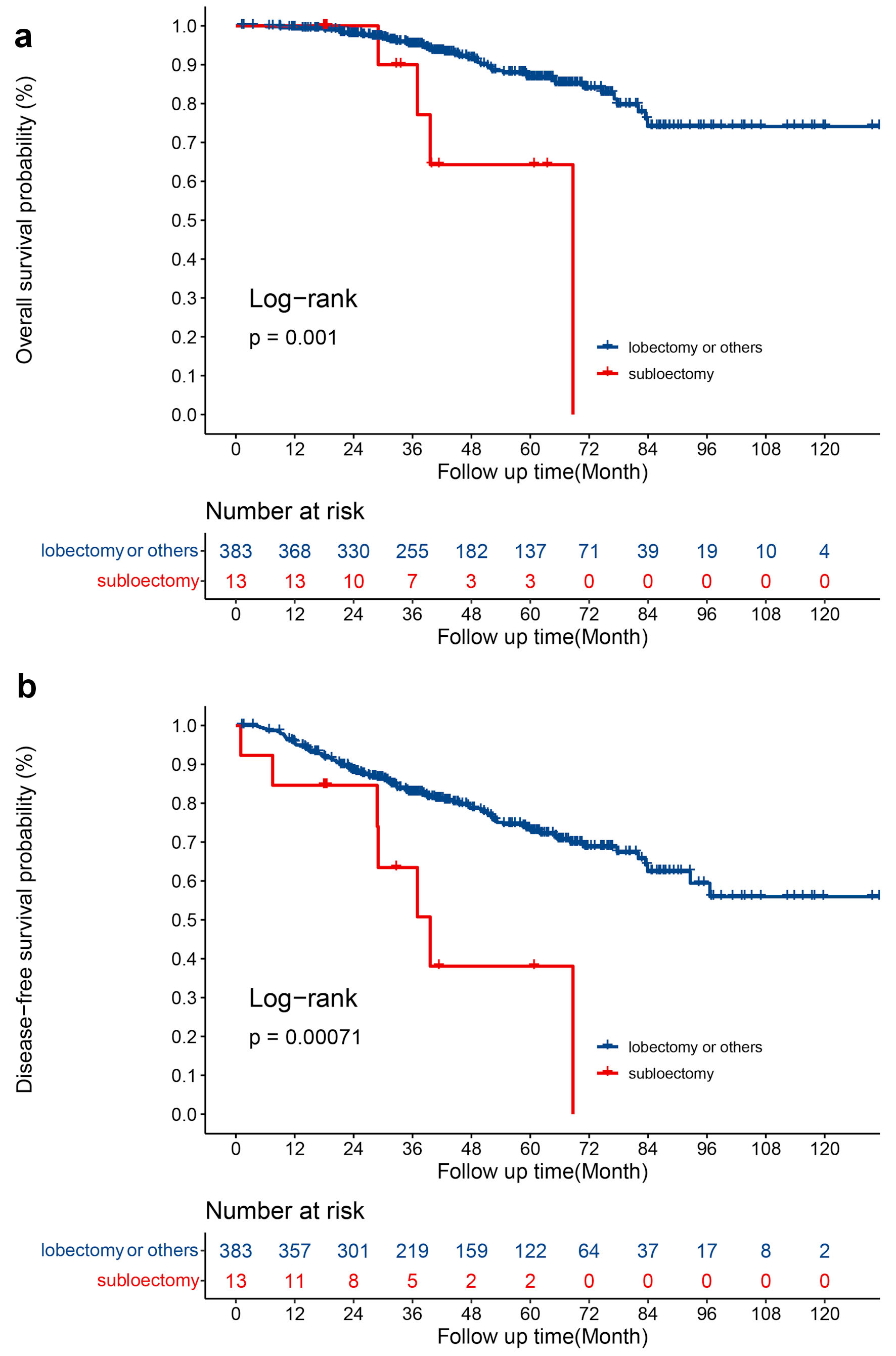 Click for large image | Figure 3. (a) OS for patients with sublobectomy and lobectomy or others. (b) DFS for patients with sublobectomy and lobectomy or others. OS: overall survival; DFS: disease-free survival. |
 Click to view | Table 4. LASSO and Cox Regression Analyses for Patients With Pure-Solid |
| Discussion | ▴Top |
Although there is consensus that the presence of GGO components is associated with better prognosis in patients with NSCLC [2-4], some questions require further study. We discovered that patients with a small proportion of GGO components (CTR > 0.75) did not get survival benefit compared with pure-solid. We also found that prognostic factors were disparate between part-GGO, and pure-solid patients while both CTR and STS were associate with outcome in patients with part-GGO.
NSCLC can be further divided into LUAD and LUSC according to morphology and immunohistochemistry. Different histological types have different molecular make-up, response to systemic therapy and prognosis [15]. Many studies indicate that a GGO on chest CT was always diagnosed as LUAD [16-18]. A GGO pathologically confirmed as LUSC is rare. Therefore, we did not review LUSC patients in this study. In addition, patients with AIS or MIA were observed to have no recurrence and the 5-year OS rate was nearly 100% [5, 6], which was already seen as positive indicator of LUAD. So, in this study, our large, homogeneous cohort only included IAC.
For part-GGO, one debate is whether CTR or STS is the indicator of prognosis. The classification of cT stage in the new staging system is according to STS [19]. The JCOG 0201 trial demonstrated that CTR can be used to predict pathological invasiveness of a nodule with 2 cm or less [20]; and the future JCOG 0804 and 0802 trials were performed according to CTR. However, Ye et al reviewed 988 clinical stage IA LUADs and found neither STS nor CTR were the prognostic factor of part-GGO [21]. In this study, we found both CTR (OS, P = 0.003) and STS (DFS, P < 0.001) were independent prognostic factors of patients with part-GGO. In addition, we found the survival of part-GGO with CTR no more than 0.25 was close to the survival of pure-GGO, while the survival of part-GGO with CTR more than 0.75 was close to the survival of pure-solid. Unlike a previous study [22], we found a small proportion of GGO component was not related with better survival in pathological stage I IAC. Based on these results, we suggested the treatment and follow-up strategies for the part-GGO with CTR more than 0.75 should be the same as for the pure-solid, and the cT classification of next TNM staging system should consider CTR as well.
VPI was seen as an important prognostic factor and regarded as a vital feature in the TNM staging system of NSCLC [23, 24]. Hattori et al retrospectively analyzed 466 patients with pathological N0 and mentioned that VPI was not a prognostic factor in part-GGO patients [25]. Fu et al reviewed 2,010 cases and supported that VPI had no prognostic value in part-GGO patients [26]. However, we revealed VPI was also an independent prognostic factor of DFS in patients with part-GGO (P = 0.002) in current study. Therefore, we should not ignore the prognostic role of VPI in patients with stage IB part-GGO, and carefully consider adjuvant therapy for them.
Sublobectomy has been wildly used for early-stage NSCLC, especially for patients with GGO components. The Japan Clinical Oncology Group (JCOG) 0804 trial has proven that sublobar resection with or without lymph node examined could provide a satisfactory local control and long-term survival in nodules with diameter less than 2 cm and CTR of 0.25 or less [27]. Cho et al reported that a nodule with a CTR of 0.25 or less after wedge resection showed an excellent prognosis [28]. A multi-center retrospective study’s findings supported sublobar resection could successfully treat the GGO-dominant nodules [29]. Results of our study also showed that sublobectomy provided similar outcomes in patients with part-GGO while the number of N2/N1station examined was not associated with prognosis. However, in patients with pure-solid nodules, sublobectomy and a smaller number of N1 station examined were associated with poor outcome. Considering wedge resection provided less extensive intraparenchymal and hilar lymph nodes’ dissection [30], sublobectomy especially wedge resection, should be cautiously adopted in pure-solid patients.
Some limitations in this study should be mentioned. First, this study is a single-center retrospective study, selection bias and recall bias might exist due to its retrospective nature. Second, spread through air space (STAS) is a negatively prognostic factor of NSCLC which associated with a GGO component [31]. However, STAS is absent in our study. Furthermore, the subtype of adenocarcinoma, which has been recognized as an important risk factor for LUAD [32], was not included in current study.
Conclusions
Though GGO component is a positively prognostic factors of patients with pathological stage I IAC, a small proportion of GGO components did not improve the survival for patients with pathological stage I IAC. Part-GGO patients with CTR more than 0.75 had similar survival with pure-solid patients. VPI, STS, and CTR are significant predictors for patients with part-GGO. For patients with pure-solid nodules, sublobar resection, especially wedge resection should be avoided.
| Supplementary Material | ▴Top |
Suppl 1. The LASSO regression analyses in entire cohort.
Suppl 2. The LASSO regression analyses in part-GGO patients.
Suppl 3. The LASSO regression analyses in pure-solid patients.
Acknowledgments
We thank Dr. Lie Zheng and Dr. Qian Cai for imaging evaluation.
Financial Disclosure
This work was supported by the Natural Science Foundation of Guangdong Province of China (grant numbers: 2019A1515011601, 2019A1515010298).
Conflict of Interest
The authors have no relevant financial or non-financial interests to disclose.
Informed Consent
The written informed consent for this retrospective study was waived due to the retrospective nature of our study.
Author Contributions
Conception and design: Jun Ye Wang and Shu Qin Dai. Provision of study materials or patients: Wen Yu Zhai, Wing Shing Wong, and Fang Fang Duan. Collection and assembly of data: Da Chuan Liang, Li Gong. Data analysis and interpretation: Wen Yu Zhai, Wing Shing Wong, and Fang Fang Duan. Manuscript writing and editing: Wen Yu Zhai, Wing Shing Wong, and Fang Fang Duan. All authors contributed to the article and approved the submitted version.
Data Availability
The key raw data have been deposited into the Research Data Deposit (http://www.researchdata.org.cn), with the approval number RDDA2022202339 and the datasets used in this study are publicly available.
Abbreviations
GGO: ground glass opacity; NSCLC: non-small cell lung cancer; AIS: adenocarcinoma in situ; MIA: minimally invasive adenocarcinoma; OS: overall survival; IAC: invasive adenocarcinoma; LUAD: lung adenocarcinoma; LUSC: lung squamous carcinoma; STS: solid tumor size; CTR: consolidation-tumor-ratio; CT: computed tomography; AJCC: American Joint Committee on Cancer; DFS: disease-free survival; VPI: visceral pleural invasion; LASSO: least absolute shrinkage and selection operator; STAS: spread through air space
| References | ▴Top |
- Austin JH, Muller NL, Friedman PJ, Hansell DM, Naidich DP, Remy-Jardin M, Webb WR, et al. Glossary of terms for CT of the lungs: recommendations of the Nomenclature Committee of the Fleischner Society. Radiology. 1996;200(2):327-331.
doi pubmed - Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Prognostic impact of a ground glass opacity component in the clinical T classification of non-small cell lung cancer. J Thorac Cardiovasc Surg. 2017;154(6):2102-2110.e2101.
doi pubmed - Hattori A, Matsunaga T, Takamochi K, Oh S, Suzuki K. Importance of ground glass opacity component in clinical stage IA radiologic invasive lung cancer. Ann Thorac Surg. 2017;104(1):313-320.
doi pubmed - Hattori A, Matsunaga T, Hayashi T, Takamochi K, Oh S, Suzuki K. Prognostic impact of the findings on thin-section computed tomography in patients with subcentimeter non-small cell lung cancer. J Thorac Oncol. 2017;12(6):954-962.
doi pubmed - Cohen JG, Reymond E, Medici M, Lederlin M, Lantuejoul S, Laurent F, Toffart AC, et al. CT-texture analysis of subsolid nodules for differentiating invasive from in-situ and minimally invasive lung adenocarcinoma subtypes. Diagn Interv Imaging. 2018;99(5):291-299.
doi pubmed - Aokage K, Miyoshi T, Ishii G, Kusumoto M, Nomura S, Katsumata S, Sekihara K, et al. Influence of ground glass opacity and the corresponding pathological findings on survival in patients with clinical stage I non-small cell lung cancer. J Thorac Oncol. 2018;13(4):533-542.
doi pubmed - Goldstraw P, Chansky K, Crowley J, Rami-Porta R, Asamura H, Eberhardt WE, Nicholson AG, et al. The IASLC lung cancer staging project: proposals for revision of the TNM stage groupings in the forthcoming (Eighth) edition of the TNM classification for lung cancer. J Thorac Oncol. 2016;11(1):39-51.
doi pubmed - Deng J, Zhao M, Wang T, She Y, Wu J, E H, Gao J, et al. A modified T categorization for part-solid lesions in Chinese patients with clinical stage I Non-small cell lung cancer. Lung Cancer. 2020;145:33-39.
doi pubmed - Su H, Dai C, Xie H, Ren Y, She Y, Kadeer X, Xie D, et al. Risk Factors of Recurrence in Patients With Clinical Stage IA Adenocarcinoma Presented as Ground-Glass Nodule. Clin Lung Cancer. 2018;19(5):e609-e617.
doi pubmed - Nakada T, Kuroda H. Narrative review of optimal prognostic radiological tools using computed tomography for T1N0-staged non-small cell lung cancer. J Thorac Dis. 2021;13(5):3171-3181.
doi pubmed - Ye T, Deng L, Xiang J, Zhang Y, Hu H, Sun Y, Li Y, et al. Predictors of Pathologic Tumor Invasion and Prognosis for Ground Glass Opacity Featured Lung Adenocarcinoma. Ann Thorac Surg. 2018;106(6):1682-1690.
doi pubmed - Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511(7511):543-550.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Yoon DW, Kim CH, Hwang S, Choi YL, Cho JH, Kim HK, Choi YS, et al. Reappraising the clinical usability of consolidation-to-tumor ratio on CT in clinical stage IA lung cancer. Insights Imaging. 2022;13(1):103.
doi pubmed - McAleese J, Taylor A, Walls GM, Hanna GG. Differential Relapse Patterns for Non-small Cell Lung Cancer Subtypes Adenocarcinoma and Squamous Cell Carcinoma: Implications for Radiation Oncology. Clin Oncol (R Coll Radiol). 2019;31(10):711-719.
doi pubmed - Arenberg D, American College of Chest P. Bronchioloalveolar lung cancer: ACCP evidence-based clinical practice guidelines (2nd edition). Chest. 2007;132(3 Suppl):306S-313S.
doi pubmed - Sawada S, Komori E, Nogami N, Segawa Y, Shinkai T, Yamashita M. Evaluation of lesions corresponding to ground-glass opacities that were resected after computed tomography follow-up examination. Lung Cancer. 2009;65(2):176-179.
doi pubmed - Sakaizawa T, Yoshizawa A, Nishimura H, Arimura T, Kobayashi N, Ozawa K, Asaka S, et al. A case of pulmonary squamous cell carcinoma revealed ground glass opacity on computed tomography. J Thorac Oncol. 2015;10(8):1229-1230.
doi pubmed - Travis WD, Asamura H, Bankier AA, Beasley MB, Detterbeck F, Flieder DB, Goo JM, et al. The IASLC lung cancer staging project: proposals for coding T categories for subsolid nodules and assessment of tumor size in part-solid tumors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol. 2016;11(8):1204-1223.
doi pubmed - Suzuki K, Koike T, Asakawa T, Kusumoto M, Asamura H, Nagai K, Tada H, et al. A prospective radiological study of thin-section computed tomography to predict pathological noninvasiveness in peripheral clinical IA lung cancer (Japan Clinical Oncology Group 0201). J Thorac Oncol. 2011;6(4):751-756.
doi pubmed - Ye T, Deng L, Wang S, Xiang J, Zhang Y, Hu H, Sun Y, et al. Lung adenocarcinomas manifesting as radiological part-solid nodules define a special clinical subtype. J Thorac Oncol. 2019;14(4):617-627.
doi pubmed - Berry MF, Gao R, Kunder CA, Backhus L, Khuong A, Kadoch M, Leung A, et al. Presence of even a small ground-glass component in lung adenocarcinoma predicts better survival. Clin Lung Cancer. 2018;19(1):e47-e51.
doi pubmed - Kawase A, Yoshida J, Miyaoka E, Asamura H, Fujii Y, Nakanishi Y, Eguchi K, et al. Visceral pleural invasion classification in non-small-cell lung cancer in the 7th edition of the tumor, node, metastasis classification for lung cancer: validation analysis based on a large-scale nationwide database. J Thorac Oncol. 2013;8(5):606-611.
doi pubmed - Yoshida J, Nagai K, Asamura H, Goya T, Koshiishi Y, Sohara Y, Eguchi K, et al. Visceral pleura invasion impact on non-small cell lung cancer patient survival: its implications for the forthcoming TNM staging based on a large-scale nation-wide database. J Thorac Oncol. 2009;4(8):959-963.
doi pubmed - Hattori A, Suzuki K, Matsunaga T, Takamochi K, Oh S. Visceral pleural invasion is not a significant prognostic factor in patients with a part-solid lung cancer. Ann Thorac Surg. 2014;98(2):433-438.
doi pubmed - Fu F, Zhang Y, Wen Z, Zheng D, Gao Z, Han H, Deng L, et al. Distinct prognostic factors in patients with stage i non-small cell lung cancer with radiologic part-solid or solid lesions. J Thorac Oncol. 2019;14(12):2133-2142.
doi pubmed - Suzuki K, Watanabe SI, Wakabayashi M, Saji H, Aokage K, Moriya Y, Yoshino I, et al. A single-arm study of sublobar resection for ground-glass opacity dominant peripheral lung cancer. J Thorac Cardiovasc Surg. 2022;163(1):289-301.e282.
doi pubmed - Cho JH, Choi YS, Kim J, Kim HK, Zo JI, Shim YM. Long-term outcomes of wedge resection for pulmonary ground-glass opacity nodules. Ann Thorac Surg. 2015;99(1):218-222.
doi pubmed - Tsutani Y, Miyata Y, Nakayama H, Okumura S, Adachi S, Yoshimura M, Okada M. Appropriate sublobar resection choice for ground glass opacity-dominant clinical stage IA lung adenocarcinoma: wedge resection or segmentectomy. Chest. 2014;145(1):66-71.
doi pubmed - Blasberg JD, Pass HI, Donington JS. Sublobar resection: a movement from the Lung Cancer Study Group. J Thorac Oncol. 2010;5(10):1583-1593.
doi pubmed - Zhong Y, Xu Y, Deng J, Wang T, Sun X, Chen D, Wu C, et al. Prognostic impact of tumour spread through air space in radiological subsolid and pure solid lung adenocarcinoma. Eur J Cardiothorac Surg. 2021;59(3):624-632.
doi pubmed - Ujiie H, Kadota K, Chaft JE, Buitrago D, Sima CS, Lee MC, Huang J, et al. Solid predominant histologic subtype in resected stage I lung adenocarcinoma is an independent predictor of early, extrathoracic, multisite recurrence and of poor postrecurrence survival. J Clin Oncol. 2015;33(26):2877-2884.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


