| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 14, Number 3, June 2023, pages 224-229
Synchronous Bilateral Breast Cancer With Discordant Receptor Status: Treating One Patient but Two Diseases
Ademola S. Ojoa, d, Adedoyin Shittua, Stacy Amadifea, Devon Jacksonb, Mica Granthamb, Ahmed Alic, Ravi Sarmac
aDepartment of Internal Medicine, Howard University Hospital, Washington, DC, USA
bDepartment of Pathology, Howard University Hospital, Washington, DC, USA
cHoward University Cancer Center, Washington, DC, USA
dCorresponding Author: Ademola S. Ojo, Department of Internal Medicine, Howard University Hospital, Washington, DC, USA
Manuscript submitted April 21, 2023, accepted June 2, 2023, published online June 11, 2023
Short title: Bilateral BC With Discordant Receptor Status
doi: https://doi.org/10.14740/wjon1603
| Abstract | ▴Top |
The expression of hormone receptors (estrogen and progesterone) and human epidermal growth factor receptor-2 (HER2) has been used for both therapeutic and prognostic purposes in the management of breast cancer. The presence of a discordant receptor status complicates the approach to treatment in patients with synchronous bilateral breast cancer. We describe the case of a 45-year-old female with synchronous bilateral breast cancer with a triple-negative tumor and a contralateral HER2-positive tumor and discussed the impact of this on the approach to therapeutic management.
Keywords: Human epidermal growth factor receptor; Estrogen receptor; Progesterone receptor; Breast cancer
| Introduction | ▴Top |
Synchronous bilateral breast cancer (SBBC) has been defined in the literature as bilateral breast cancer foci diagnosed simultaneously with a cut-off of within 3 and 6 months [1]. It represents 0.2-3% of all newly diagnosed breast cancer cases and is associated with higher mortality compared to unilateral breast cancer [1, 2]. Similar genetic predisposition, environmental exposure, or unrelated genetic or epigenetic alterations underlie the development of SBBC [3]. Tumor heterogeneity, regarded as the hallmark of malignancies, occurs in SBBC with a significant impact on therapeutic management [4]. This heterogeneity is clinically assessed by various modalities including genetic profiling of tumors and assessment of receptor expression status [5].
The expression of hormone receptors (estrogen and progesterone) and human epidermal growth factor receptor-2 (HER2) has been used for both therapeutic and prognostic purposes in the management of breast cancer. We present a case of SBBC with heterogenous receptor expression and explore its impact on therapeutic management.
| Case Report | ▴Top |
Investigations
This case details a 45-year-old Hispanic woman who was referred to our clinic by her primary care physician after she discovered a lump in her left breast. The patient reported that the lump was painless, about the size of a pea but denied any nipple discharge or breast skin changes. Her past medical history included type 2 diabetes for which she takes metformin. Her family history is significant for breast cancer in her mother in her early 50s and three other maternal cousins.
On physical examination, she had a palpable, freely movable, non-tender mass in the left breast upper outer quadrant with no skin, nipple, or areolar changes. There was no palpable mass in the right breast and no palpable axillary lymphadenopathy bilaterally.
Diagnosis
Bilateral diagnostic mammography showed a 1.2 × 1.9 cm right breast mass (upper inner quadrant) and a 1.9 × 2.1 cm left breast mass (upper outer quadrant). Breast ultrasound revealed a 1.3 × 1.6 cm heterogenous mass in the right breast with normal appearing right axillary nodes and a 1.8 × 2.1 cm solid mass in the left breast with an abnormal appearing left axillary node. Ultrasound-guided biopsy of the breast lesions showed invasive, poorly differentiated, high-grade ductal carcinoma with medullary features in the right breast, and poorly differentiated invasive ductal carcinoma in the left breast (Figs. 1, 2). The right breast lesion was negative for estrogen receptor (ER-) and progesterone receptor (PR-); the HER2/neu immunohistochemistry (IHC) score was 2+ but was positive by fluorescence in situ hybridisation (FISH) (percentage of positive nuclei > 90%). The left breast lesion was negative for ER, PR, and HER2 expression (triple-negative breast cancer (TNBC)). Staging workup with positron emission tomography/computed tomography (PET/CT) confirmed the lesions with a maximum standardized uptake value (SUV) of 6.2 in the right breast lesion and a left breast lesion with a maximum SUV of 17.6 in addition to a hypermetabolic left axillary lymph node with an SUV of 6.0 (Fig. 3). Genetic testing showed the presence of mutation in BRCA1 gene.
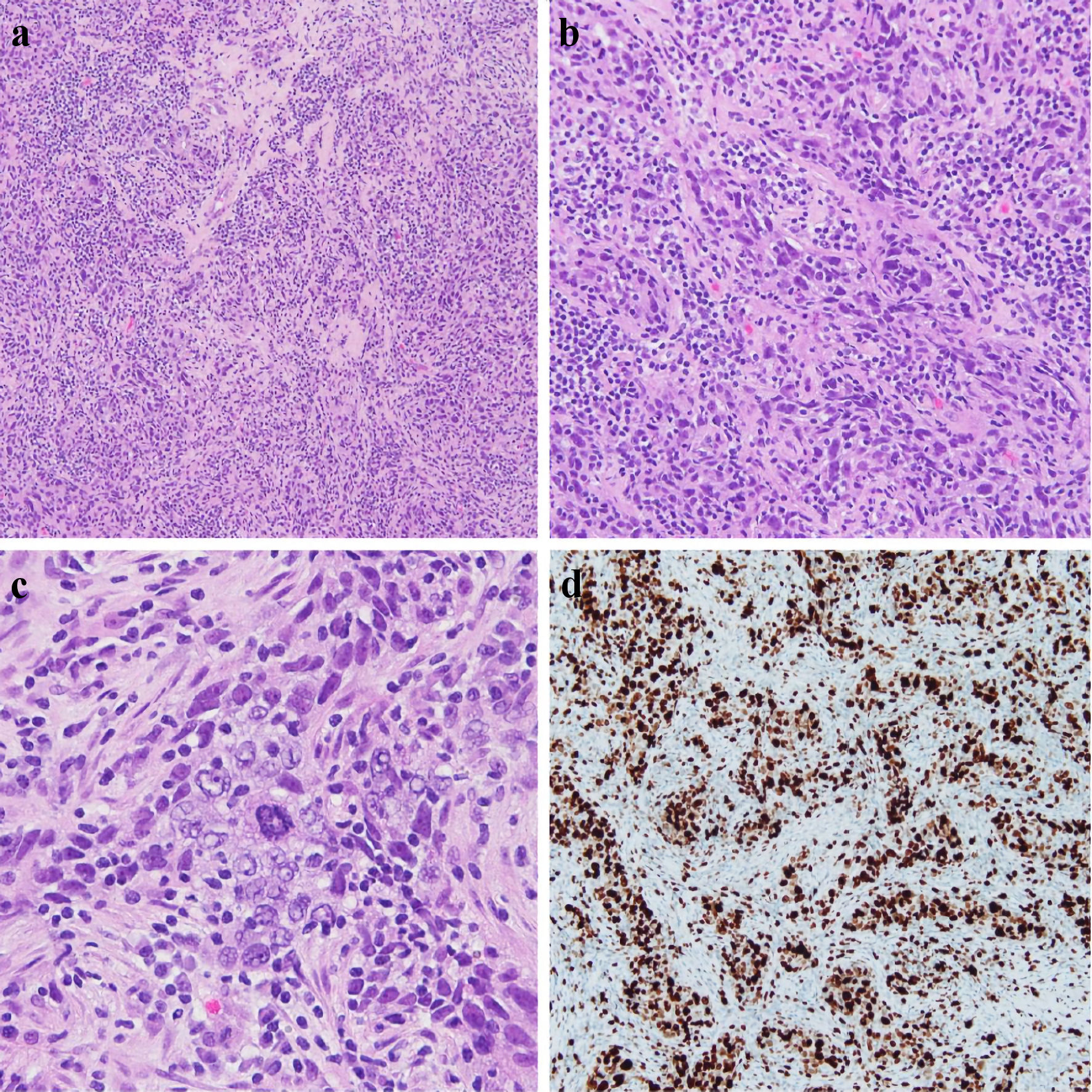 Click for large image | Figure 1. Right core breast biopsy (a, × 10), (b, × 20), (c, × 40) showing infiltrating tumor cells with large and atypical nuclei, a syncytial growth pattern, and a background of lymphocytes. An atypical mitotic figure is present in the center of (c). Ki67 IHC stain showing > 90% nuclear staining of tumor cells, reflecting increased proliferation (d). IHC: immunohistochemistry. |
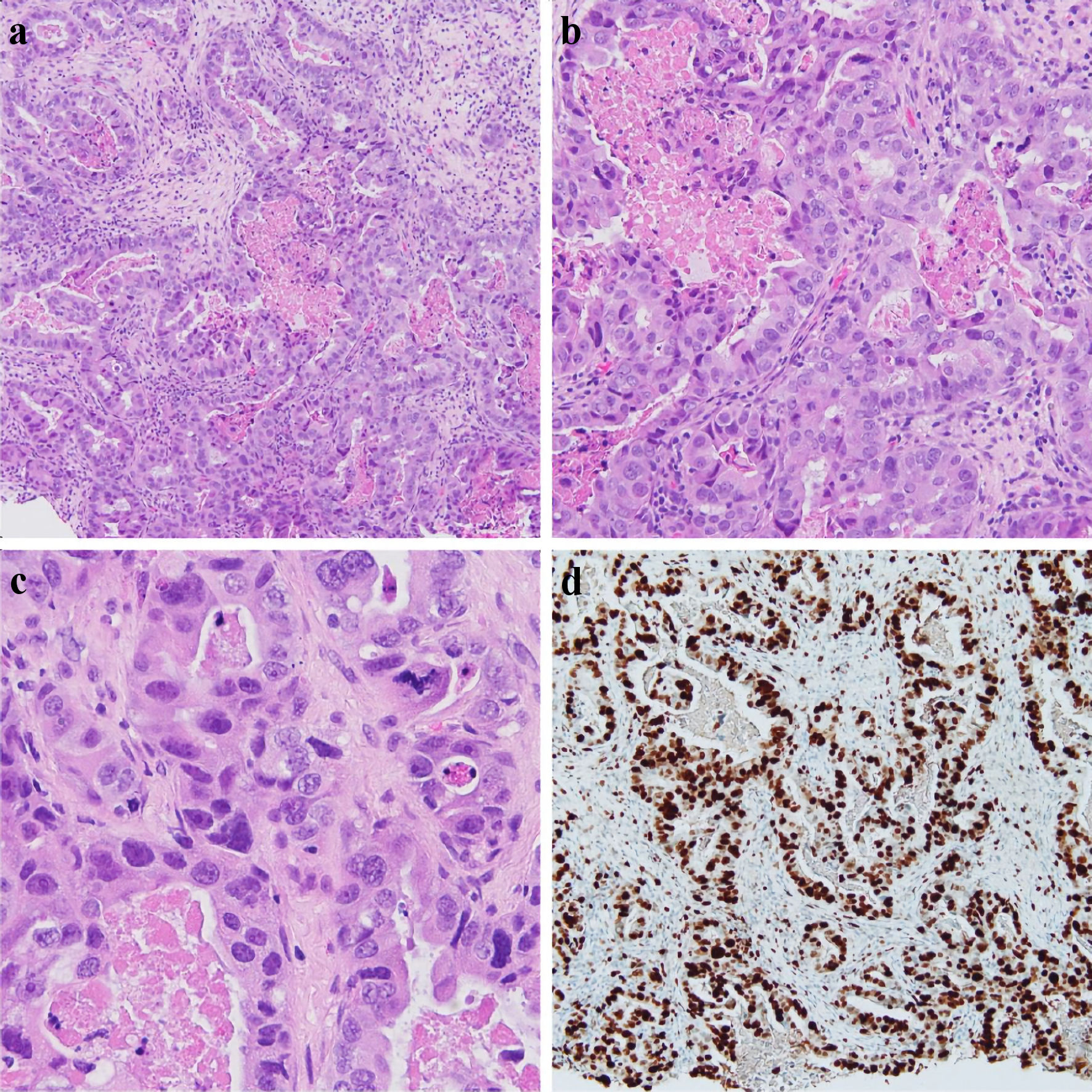 Click for large image | Figure 2. Left breast core biopsy (a, × 10), (b, × 20), (c, × 40) showing infiltrating tumor cells with large, pleomorphic nuclei, with a glandular/tubule formation and associated necrosis within the tubules. An atypical mitotic figure can be seen in (c). Ki67 IHC stain showing > 90% nuclear staining of tumor cells, reflecting increased proliferation (d). IHC: immunohistochemistry. |
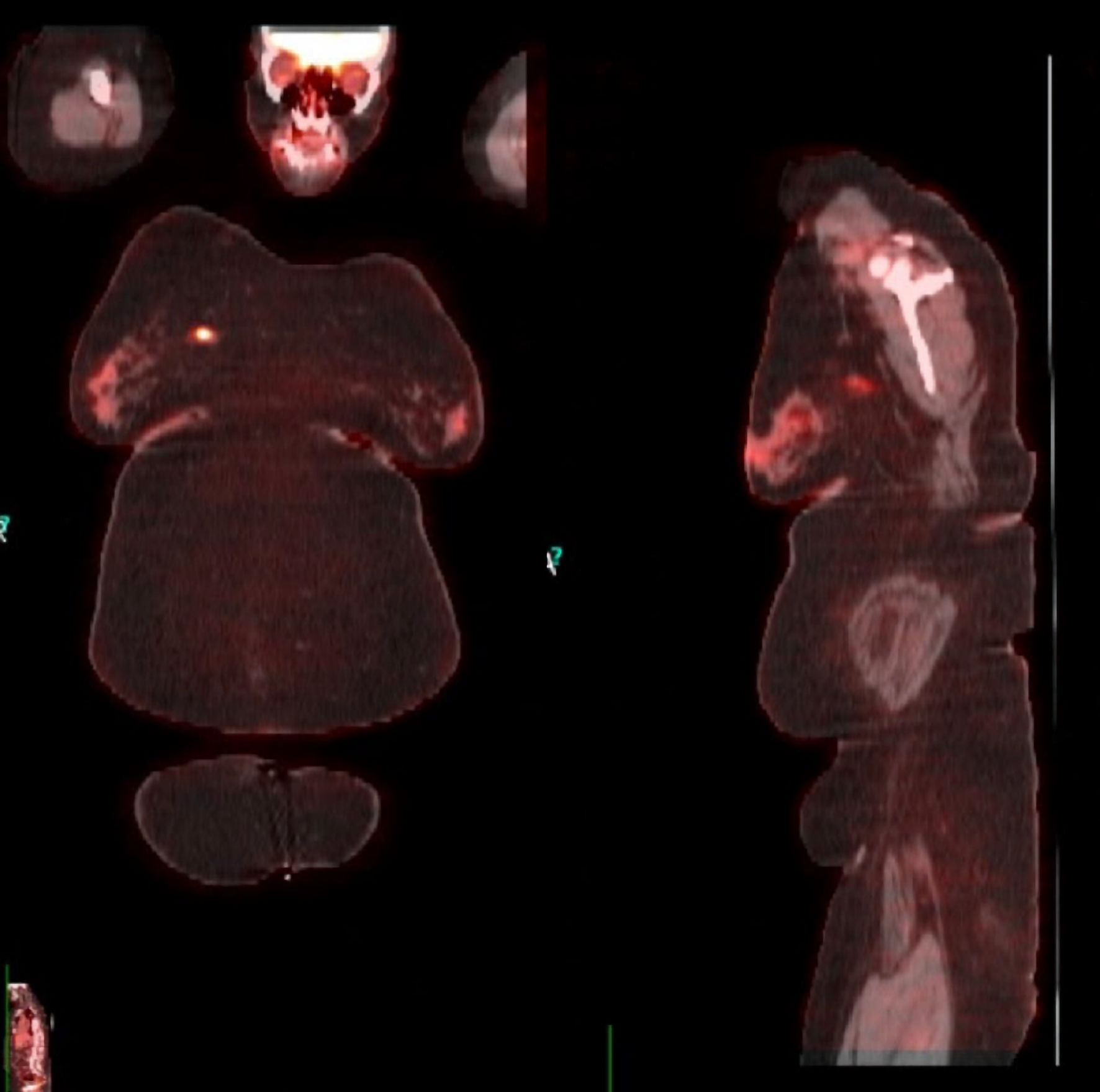 Click for large image | Figure 3. PET/CT scan for staging confirming the lesions in both breasts. PET/CT: positron emission tomography/computed tomography. |
Treatment
She received neoadjuvant therapy with docetaxel/carboplatin/trastuzumab/pertuzumab (TCHP) every 3 weeks for six cycles followed by adriamycin/cyclophosphamide/pembrolizumab every 3 weeks for four cycles.
Follow-up and outcomes
Follow-up imaging showed a complete response to neoadjuvant therapy with no residual mass in the breasts or axillary lymph node abnormalities. She subsequently underwent a bilateral total mastectomy with sentinel lymph node biopsy and immediate tissue expander reconstruction. Histopathology of resected specimens showed a complete response with no evidence of primary tumor in the breasts or axillary node metastasis (ypT0ypN0 in both left and right breast). Pembrolizumab was resumed postoperatively. Although the patient did not immediately meet the criteria for post-mastectomy radiation treatment (PMRT), she was scheduled for evaluation for possible radiation treatment after the completion of breast reconstruction with the removal of tissue expanders. She was referred for gynecologic evaluation for possible prophylactic oophorectomy given her BRCA1 gene mutation. She is 15 months post-diagnosis and remains disease free.
| Discussion | ▴Top |
Here, we present a case of SBBC with heterogeneity in receptor expression. Although this patient initially presented with a left breast lump, the presence of bilateral lesions was confirmed on imaging studies with biopsy establishing the diagnosis of a left TNBC and a right breast HER2-positive disease. Most second synchronous tumors are detected by imaging studies in patients who presented with a unilateral breast lesion, and they usually share similar histological features [6]. Some of the factors associated with the development of bilateral tumors include BRCA mutations, younger individuals, family history of bilateral breast cancer, and lobular histology subtype [7]. Although these tumors are often similar histologically, heterogeneity can occur between different tumors (intertumor heterogeneity) [8]. These differences in the genomic and phenotypic properties exhibited by different tumors in the same individual often arise from diverse metabolic, immunological, and trophic factors that work to create different neoplastic microenvironments [8, 9].
The BRCA1 mutation found in our patient is a tumor suppressor gene located on chromosome 17 that plays a role in DNA damage repair [10]. The lifetime risk of breast cancer in these individuals is high, up to 72% [11]. BRCA1 mutation is associated with the development of TNBC [12]. Our patient has a TNBC in addition to a contralateral HER2-positive tumor. Individuals with BRCA mutations who develop TNBC are sensitive to DNA-damaging chemotherapeutic agents, although this does not translate to improved survival [13, 14].
Although our patient had an early-stage disease bilaterally, she was treated with neoadjuvant therapy before undergoing bilateral mastectomy. Neoadjuvant therapy was traditionally used to downstage locally advanced or inoperable tumors to improve surgical outcomes. However, neoadjuvant therapy is being increasingly used in early-stage disease to assess tumor response and to guide future adjuvant therapies [15]. In addition, it creates a time window for planning breast reconstruction if a patient chooses to undergo a mastectomy. In patients with TNBC and HER2-positive breast cancer, response to chemotherapy is a strong predictor of recurrence [16, 17]. As such, response to neoadjuvant chemotherapy provides a real-life validation model for predicting the long-term effect of treatment [18]. Although the clinical utility of neoadjuvant chemotherapy has been demonstrated in studies, in terms of long-term outcomes, there is insufficient evidence from clinical trials on the superiority of pre- versus post-mastectomy chemotherapy [19, 20].
Our approach to neoadjuvant therapy was the use of two lines of chemo-/targeted therapy. The initial regimen was the TCHP, which not only provided HER2-directed therapy but also contained an anthracycline-free platinum plus taxane regimen for her TNBC. This was followed by a neoadjuvant pembrolizumab-chemotherapy (doxorubicin-cyclophosphamide) regimen specifically directed at the TNBC. One major consideration for this approach was the risk of cardiotoxicity from trastuzumab and adriamycin. Administering trastuzumab first allows patient to receive a full complement of HER2-directed therapy prior to initiation of adriamycin for TNBC. Trastuzumab cardiotoxicity is largely reversible and non-progressive, while adriamycin toxicity is progressive, cumulative dose-dependent and irreversible [21, 22]. In the KEYNOTE 522 trial, neoadjuvant pembrolizumab plus chemotherapy followed by adjuvant pembrolizumab demonstrated a superior event-free survival than chemotherapy alone in TNBC [23]. Although immune checkpoint inhibitors have demonstrated success in the treatment of melanoma, lung cancer and microsatellite unstable colon cancer, the efficacy in breast cancer is somewhat limited [24]. This is likely due to the low expression of programmed cell death-ligand 1 (PD-L1) in breast cancer (10-30%), although this varies by tumor stage and subtype, with the highest expression in TNBC (30-60%) [25, 26]. Our patient had a pathological complete response (pCR) bilaterally. A pCR has been shown to be associated with prolonged event-free survival and overall survival in TNBC [27].
PMRT has been shown to reduce the rate of locoregional recurrence and improve long-term outcomes in selected patient populations [28, 29]. However, the choice of who receives adjuvant radiation treatment after mastectomy depends on the risk of disease recurrence. In patients who received neoadjuvant therapy, the presence of macroscopic nodal disease after treatment is a strong predictor of a higher rate of recurrence after mastectomy [30]. Such individuals in addition to those who have residual breast disease after mastectomy are candidates for PMRT. Although data from prospective studies on the benefit of adjuvant PMRT in patients who achieved a complete response to neoadjuvant chemotherapy are lacking, evidence from retrospective studies is conflicting. For instance, a retrospective study of PMRT in patients with stage III and IV disease found a lower 10-year rate of locoregional recurrence in those who achieved pCR with neoadjuvant chemotherapy compared to those who did not [31]. In contrast, another retrospective study of 3,000 women did not find a significant difference in recurrence rate with or without PMRT [32]. In that study, the predictors of recurrence were pre-treatment nodal involvement, tumor size > 5 cm, and the presence of residual disease after neoadjuvant chemotherapy. As such, an individualized approach to PMRT is warranted, which should take into consideration the risk of disease recurrence, radiation-related morbidities, and the patient’s wishes.
Learning points
A heterogeneity of receptor status adds a layer of complexity to the therapeutic management of SBBC. Each of the tumors should be treated as a distinct entity and the therapeutic approach should be guided by the optimal chance of achieving a pCR in both tumors and improving long-term outcomes.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Informed consent was obtained from the patient for publication of this case report and the accompanying images.
Author Contributions
AO, AS, SA, and RS conceived and designed the study. AO, SA, DJ, MG and AA collected and interpreted all relevant clinical and laboratory data. AO, AA and RS prepared the manuscript. All authors read and approved the final manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Holm M, Tjonneland A, Balslev E, Kroman N. Prognosis of synchronous bilateral breast cancer: a review and meta-analysis of observational studies. Breast Cancer Res Treat. 2014;146(3):461-475.
doi pubmed - McCaul KA. Bilateral breast cancer incidence and survival. [PhD thesis]. North Terrace, ADELAIDE SA 5005: University of Adelaide; 2006. Available from: University of Adelaide, School of Population Health and Clinical Practice, Library E-Reserve. https://digital.library.adelaide.edu.au/dspace/bitstream/2440/37870/8/02whole.pdf.
- Dawson PJ, Maloney T, Gimotty P, Juneau P, Ownby H, Wolman SR. Bilateral breast cancer: one disease or two? Breast Cancer Res Treat. 1991;19(3):233-244.
doi pubmed - Turashvili G, Brogi E. Tumor heterogeneity in breast cancer. Front Med (Lausanne). 2017;4:227.
doi pubmed pmc - Song JL, Chen C, Yuan JP, Sun SR. Progress in the clinical detection of heterogeneity in breast cancer. Cancer Med. 2016;5(12):3475-3488.
doi pubmed pmc - Hungness ES, Safa M, Shaughnessy EA, Aron BS, Gazder PA, Hawkins HH, Lower EE, et al. Bilateral synchronous breast cancer: mode of detection and comparison of histologic features between the 2 breasts. Surgery. 2000;128(4):702-707.
doi pubmed - Hartman M, Czene K, Reilly M, Adolfsson J, Bergh J, Adami HO, Dickman PW, et al. Incidence and prognosis of synchronous and metachronous bilateral breast cancer. J Clin Oncol. 2007;25(27):4210-4216.
doi pubmed - Liu J, Dang H, Wang XW. The significance of intertumor and intratumor heterogeneity in liver cancer. Exp Mol Med. 2018;50(1):e416.
doi pubmed pmc - Vitale I, Shema E, Loi S, Galluzzi L. Intratumoral heterogeneity in cancer progression and response to immunotherapy. Nat Med. 2021;27(2):212-224.
doi pubmed - McCrorie AD, Ashfield S, Begley A, McIlmunn C, Morrison PJ, Boyd C, Eccles B, et al. Multifocal breast cancers are more prevalent in BRCA2 versus BRCA1 mutation carriers. J Pathol Clin Res. 2020;6(2):146-153.
doi pubmed pmc - Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips KA, Mooij TM, Roos-Blom MJ, Jervis S, et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402-2416.
doi pubmed - De Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A, Bodmer A, et al. Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020;10(1):7073.
doi pubmed pmc - De Talhouet S, Peron J, Vuilleumier A, Friedlaender A, Viassolo V, Ayme A, Bodmer A, et al. Publisher Correction: Clinical outcome of breast cancer in carriers of BRCA1 and BRCA2 mutations according to molecular subtypes. Sci Rep. 2020;10(1):19248.
doi pubmed pmc - Wang C, Zhang J, Wang Y, Ouyang T, Li J, Wang T, Fan Z, et al. Prevalence of BRCA1 mutations and responses to neoadjuvant chemotherapy among BRCA1 carriers and non-carriers with triple-negative breast cancer. Ann Oncol. 2015;26(3):523-528.
doi pubmed - Montemurro F, Nuzzolese I, Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer? Expert Opin Pharmacother. 2020;21(9):1071-1082.
doi pubmed - Teshome M, Hunt KK. Neoadjuvant therapy in the treatment of breast cancer. Surg Oncol Clin N Am. 2014;23(3):505-523.
doi pubmed pmc - Sulpher J, Dent R, Dent S. Neoadjuvant chemotherapy in breast cancer: what questions remain? Curr Opin Support Palliat Care. 2014;8(1):59-63.
doi pubmed - Asaoka M, Gandhi S, Ishikawa T, Takabe K. Neoadjuvant chemotherapy for breast cancer: past, present, and future. Breast Cancer (Auckl). 2020;14:1178223420980377.
doi pubmed pmc - Vaid AK, Khurana A, Sharma D, Gautam D, Wadhwa J, Agarwal R, Kaur K, et al. Clinical characteristics and outcome trends of adjuvant anthracycline and taxane regimen for early stage breast cancer. World J Oncol. 2020;11(3):106-111.
doi pubmed pmc - Mieog JS, van der Hage JA, van de Velde CJ. Preoperative chemotherapy for women with operable breast cancer. Cochrane Database Syst Rev. 2007;2007(2):CD005002.
doi pubmed pmc - Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ. Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol. 2005;23(31):7820-7826.
doi pubmed - Rawat PS, Jaiswal A, Khurana A, Bhatti JS, Navik U. Doxorubicin-induced cardiotoxicity: An update on the molecular mechanism and novel therapeutic strategies for effective management. Biomed Pharmacother. 2021;139:111708.
doi pubmed - Schmid P, Cortes J, Dent R, Pusztai L, McArthur H, Kummel S, Bergh J, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. N Engl J Med. 2022;386(6):556-567.
doi pubmed - Tokumaru Y, Joyce D, Takabe K. Current status and limitations of immunotherapy for breast cancer. Surgery. 2020;167(3):628-630.
doi pubmed pmc - Hong M, Kim JW, Kim MK, Chung BW, Ahn SK. Programmed cell death-ligand 1 expression in stromal immune cells is a marker of breast cancer outcome. J Cancer. 2020;11(24):7246-7252.
doi pubmed pmc - Zhang M, Sun H, Zhao S, Wang Y, Pu H, Wang Y, Zhang Q. Expression of PD-L1 and prognosis in breast cancer: a meta-analysis. Oncotarget. 2017;8(19):31347-31354.
doi pubmed pmc - Sikov WM, Polley M-Y, Twohy E, et al. CALGB (Alliance) 40603: long-term outcomes (LTOs) after neoadjuvant chemotherapy (NACT) +/− carboplatin (Cb) and bevacizumab (Bev) in triple-negative breast cancer (TNBC). J Clin Oncol. 2019;37(Suppl 15):591-591.
doi - Clarke M, Collins R, Darby S, Davies C, Elphinstone P, Evans V, Godwin J, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet. 2005;366(9503):2087-2106.
doi pubmed - Danish Breast Cancer Cooperative Group, Nielsen HM, Overgaard M, Grau C, Jensen AR, Overgaard J. Study of failure pattern among high-risk breast cancer patients with or without postmastectomy radiotherapy in addition to adjuvant systemic therapy: long-term results from the Danish Breast Cancer Cooperative Group DBCG 82 b and c randomized studies. J Clin Oncol. 2006;24(15):2268-2275.
doi pubmed - Liu J, Mao K, Jiang S, Jiang W, Chen K, Kim BY, Liu Q, et al. The role of postmastectomy radiotherapy in clinically node-positive, stage II-III breast cancer patients with pathological negative nodes after neoadjuvant chemotherapy: an analysis from the NCDB. Oncotarget. 2016;7(17):24848-24859.
doi pubmed pmc - Buchholz TA, Tucker SL, Masullo L, Kuerer HM, Erwin J, Salas J, Frye D, et al. Predictors of local-regional recurrence after neoadjuvant chemotherapy and mastectomy without radiation. J Clin Oncol. 2002;20(1):17-23.
doi pubmed - Mamounas EP, Anderson SJ, Dignam JJ, Bear HD, Julian TB, Geyer CE, Jr., Taghian A, et al. Predictors of locoregional recurrence after neoadjuvant chemotherapy: results from combined analysis of National Surgical Adjuvant Breast and Bowel Project B-18 and B-27. J Clin Oncol. 2012;30(32):3960-3966.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


