| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 5, October 2023, pages 325-339
Folfirinox vs. Gemcitabine + Nab-Paclitaxel as the First-Line Treatment for Pancreatic Cancer: A Systematic Review and Meta-Analysis
Nooraldin Merzaa, m , Sabeeh Khawar Farooquib, Sophia Haroon Darc, Tony Varughesed, Rehmat Ullah Awane, Lamaan Qureshif, Saad Ali Ansarig, Hadi Qureshih, Jamie Mcilvainei, Ishaan Vohraj, Yusuf Nawrask, Abdallah Kobeissyl, Mona Hassanl
aDepartment of Internal Medicine, University of Toledo, Toledo, OH, USA
bDepartment of Medicine, Ziauddin Medical University, Karachi, Pakistan
cDepartment of Internal Medicine, Long Island Jewish Medical Center-Northshore University Hospital, Manhasset, NY, USA
dDepartment of Internal Medicine, Hackensack University Medical Center, Hackensack, NJ, USA
eDepartment of Internal Medicine, Ochsner Rush Hospital, Meridian, MS, USA
fEdson College of Nursing and Health Innovations, Tempe, AZ, USA
gDepartment of Internal Medicine, University of California, Riverside School of Medicine, Riverside, CA, USA
hSchool of Liberal Arts, Arizona State University, Maricopa, AZ, USA
iDepartment of OBGYN-Rutgers Jersey City, Jersey City, NJ, USA
jGastroenterology Department, University of Illinois, Peoria, IL, USA
kUniversity of Toledo College of Medicine and Life Sciences, Toledo, OH, USA
lDepartment of Gastroenterology, University of Toledo, Toledo, OH, USA
mCorresponding Author: Nooraldin Merza, Department of Internal Medicine, University of Toledo, Toledo, OH, USA
Manuscript submitted April 22, 2023, accepted July 7, 2023, published online September 20, 2023
Short title: FFX vs. GnP Treatment for PC
doi: https://doi.org/10.14740/wjon1604
| Abstract | ▴Top |
Background: The efficacy and safety of Folfirinox (FFX) or gemcitabine + nab-paclitaxel (GnP) to be used as the first-line drugs for pancreatic cancer (PC) is yet to be established. We conducted an analysis of retrospective studies to assess the efficacy and safety of these two regimens by comparing their survival and safety outcomes in patients with PC.
Methods: We conducted an extensive review of two electronic databases from inception till February 2023 to include all the relevant studies that compared FFX with GnP published and unpublished work. Retrospective studies were only included. Overall survival (OS) and progression-free survival (PFS) were pooled using hazard ratios (HRs), while objective response rate (ORR) and safety outcomes were pooled using odds ratios (ORs) with 95% confidence interval (CI) using the random effects model.
Results: A total of 7,030 patients were identified in a total of 21 articles that were shortlisted. Pooled results concluded that neither FFX nor GnP was associated to increase the OS time (HR: 0.93, 95% CI: 0.83 - 1.04; P = 0.0001); however, FFX was more likely associated with increased PFS when compared to GnP (HR: 0.88, 95% CI: 0.81 - 0.97; P < 0.0001). ORR proved to be non-significant between the two regimens (OR: 0.90, 95% CI: 0.64 - 1.27; P = 0.15). Safety outcomes included neutropenia, anemia, thrombocytopenia and diarrhea. GnP was more associated with diarrhea (OR: 1.96, 95% CI: 1.22 - 3.15; P = 0.001), while FFX was seen to cause anemia (OR: 0.70, 95% CI: 0.51 - 0.98; P = 0.10) in PC patients. Neutropenia and thrombocytopenia were in-significant in the two drug regimens (OR: 1.10, 95% CI: 0.92 - 1.31; P = 0.33 and OR: 0.83, 95% CI: 0.60 - 1.13; P = 0.23, respectively).
Conclusion: FFX and GnP showed a significant difference in increasing the PFS, while no difference was observed while measuring OS. Safety outcomes showed that FFX and GnP shared similar safety profiles as FFX was associated with hematological outcomes, while GnP was more associated with non-hematological outcomes.
Keywords: Folfirinox; Gemcitabine + nab-paclitaxel; Pancreatic cancer; Overall survival; Progression-free survival; Objective response rate
| Introduction | ▴Top |
Pancreatic cancer (PC) is considered as the root cause of cancer-related deaths worldwide with poor prognosis and lowest 5-year survival outcome [1, 2]. Thirty to forty percent of people are diagnosed after the age of 75 years with median age of diagnosis falling at 70 years [3-5]. Since 1990s numerous attempts have been made to treat PC [6-8]. After the conduction of two large scaled trials, two drug regimens have been introduced to treat PC as the first-line treatment.
The gemcitabine + nab-paclitaxel (GnP) combination was initiated in the market after its promising effects on the overall survival (OS) and progression-free survival (PFS) when compared to the gemcitabine monotherapy in the MPACT trial (Fig. 1) [7]. Similarly, PRODIGE4/ACCORD11 trial introduced Folfirinox (FFX), a combination of 5-fluorouracil (5-FU), leucovorin, oxaliplatin, and irinotecan, which showed significant results with regards to survival rates and benefits in contrast to gemcitabine being used alone [9]. As a consequence, two regimens are now in market to be used as first-line drugs to treat PC.
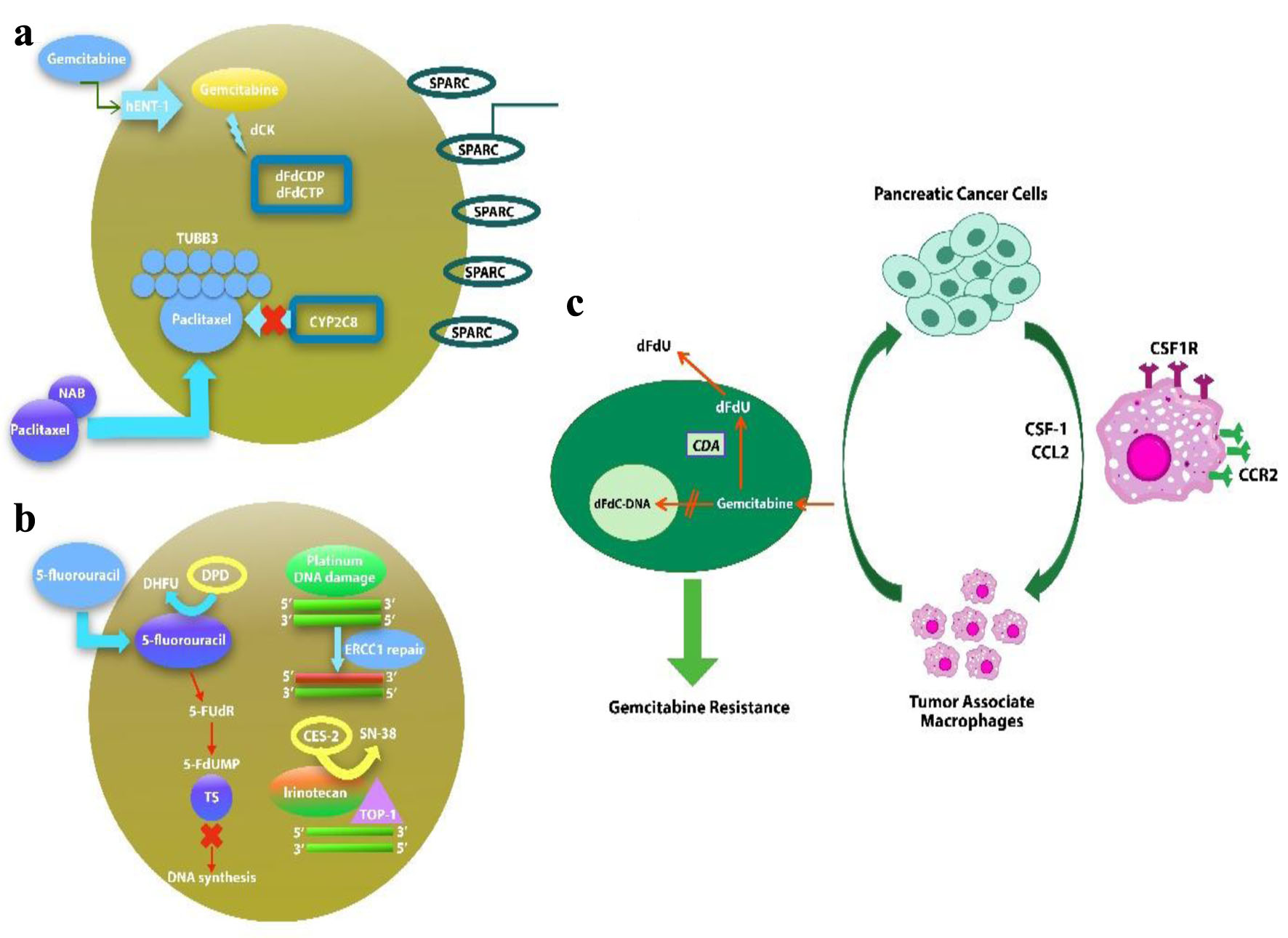 Click for large image | Figure 1. Potential biomarkers in nab-paclitaxel/gemcitabine (a), Folfirinox (b) regimens of drug metabolism and mechanism of action. (c) Macrophages induce gemcitabine resistance by up-regulation of CDA. |
Despite the introduction of the two regimens, there is a lack of data when comparing the two combinations exclusively with respect to OS, PFS and objective response rate (ORR). As concluded by a previous meta-analysis, the two regimens had no association in increasing the OS, PFS or ORR. Furthermore, the previous studies did not evaluate the detailed safety profiles for the two regimens; hence, our meta-analysis, with a larger sample size and excellent statistical power, examined the safety profiles and included the most recent published studies. Importantly, the literature search period for our study extends until February 2023; therefore, our study encompasses recently published randomized controlled trials (RCTs), which were conducted to fill in the literature gap [10]. Lastly, our study has different clinical implications based on patient settings. In practice, FFX is often recommended for younger patients, while GnP is typically preferred in older patients. This aspect adds another layer of complexity in the direct comparison of these two regimens, which was duly considered in our study [11, 12]. We conducted this meta-analysis and systematic review to rule out all the in-indifferences and find the best therapy in regard to efficacy and toxicity to be used as first-line treatment for PC in all age groups.
| Materials and Methods | ▴Top |
Data sources and search strategy
This meta-analysis was conducted in accordance to the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) guidelines [13]. A comprehensive review of the two electronic databases was conducted from inception till February 2023 independently by two researchers which included MEDLINE and Cochrane Central incorporating articles written in English only. We utilized the following search string: (Metastatic Pancreatic cancer OR Pancreatic carcinoma OR Pancreatic cancer OR Pancreatic tumor OR Pancreatic neoplasm OR Pancreatic adenocarcinoma OR Pancreatic adenoma) AND (Fluorouracil OR Leucovorin OR Irinotecan OR Oxaliplatin OR Folfirinox OR Folfirinox) AND (Gemcitabine plus nab-paclitaxel OR GnP OR albumin bound paclitaxel OR Gemcitabine) AND (Progression free survival OR Overall survival OR OS OR PFS OR Toxicity). In addition, generic and trade names were used to screen any published or unpublished work at [14, 15] that could have been left out. Lastly, grey literature and reference list from past meta-analysis were manually reviewed to rule out the chances of missing any study (Supplementary Material 1, www.wjon.org).
Study selection
The eligibility criteria to shortlist the studies were as follows: 1) Retrospective studies published in English; 2) Studies conducted on human species; and 3) Studies reporting adverse effects or safety in either metastatic PC or locally advanced metastatic cancer. The standard dose of FFX included oxaliplatin 85 mg/m2, leucovorin 400 mg/m2, irinotecan 180 mg/m2,5-FU bolus 400 mg/m2 and 5-FU 2,400 mg/m2. Case reports, case series and meta-analysis were left out with duplicates to remove any inaccuracies.
Data extraction and assessment of study quality
Our systematic search was supplemented by a manual search with results being exported to MENDLEY reference library to pull out any duplicates. The left-over studies were assessed carefully by two independent researchers (NM and SD). The inclusion of the study was made on the lines of previously defined criteria, while a third reviewer was consulted AN to resolve any discrepancies. Studies were shortlisted first on the basis of their title and abstract after which a full text review was given to confirm its inclusion. From the final list of studies, primary outcome of OS and PFS was extracted along with any grade adverse events (according to the National Cancer Institute Common Terminology Criteria for Adverse events) including hematological and non-hematological outcomes such as neutropenia, thrombocytopenia, anemia and diarrhea.
Statistical analysis
Review manager version 5.4.1 was used to conduct all the statistical analysis. The results were pooled as hazard ratios (HRs) for OS and PFS, while odds ratios (ORs) were used to pool the results for ORR and adverse events (AEs) with a 95% confidence interval (CI) using the random effects model. Forest plots were made to evaluate the results. Heterogeneity across all the studies was based on the Higgins I2 statistics with I2 = 25-50% considered mild, I2 = 50-75% moderate and I2 > 75% severe heterogeneity. Visual screening of the funnel plots was done to eradicate publication bias. A P-value of less than 0.05 was considered to be significant in all the cases.
| Results | ▴Top |
Literature search results
Our initial search of two electronic databases yielded a total of 1,127 potentially relevant articles. After screening and exclusion of articles, 21 studies remained for synthetic analysis. Our analysis included a total of two drugs of FFX and GnP. PRISMA flow chart below summarizes the results of our literature search (Supplementary Material 2, www.wjon.org). Graphical representation of the study in a systematic way is shown in Supplementary Material 3 (www.wjon.org).
Study characteristics, sample demography, and quality assessment
A total of 7,030 patients were found in the shortlisted studies with 3,653 patients in the FFX group while 3,377 patients in the GnP group. Median follow-up duration of studies ranged from 4.1 to 33 months. The relevant study and patient characteristics are found in Table 1 while Table 2 shows the demographic details [16-36]. Table 3 shows the study criteria and guidelines [16-36]. All the studies included had high quality. A comprehensive quality assessment of each study is presented in Supplementary Material 4 (www.wjon.org). The tumor locations are presented in Supplementary Material 5 (www.wjon.org).
 Click to view | Table 1. Study and Patient Characteristics |
 Click to view | Table 2. Demographic Details of the Study and Patient Characteristics |
 Click to view | Table 3. Study Criteria and Guidelines |
OS
A total of 18 studies [16-33] reported OS out of the 21 relevant studies. There was no statistical difference found in between the usage of FFX and GnP as first-line treatment to increase the OS time (HR: 0.93, 95% CI: 0.83 - 1.04; P = 0.0001) (Fig. 2a, b). Sensitivity analysis was conducted and studies with different guidelines were removed, although no significant difference was observed in the results (HR: 0.91; 95% CI: 0.81 - 1.01; P = 0.005) (Supplementary Material 6, www.wjon.org).
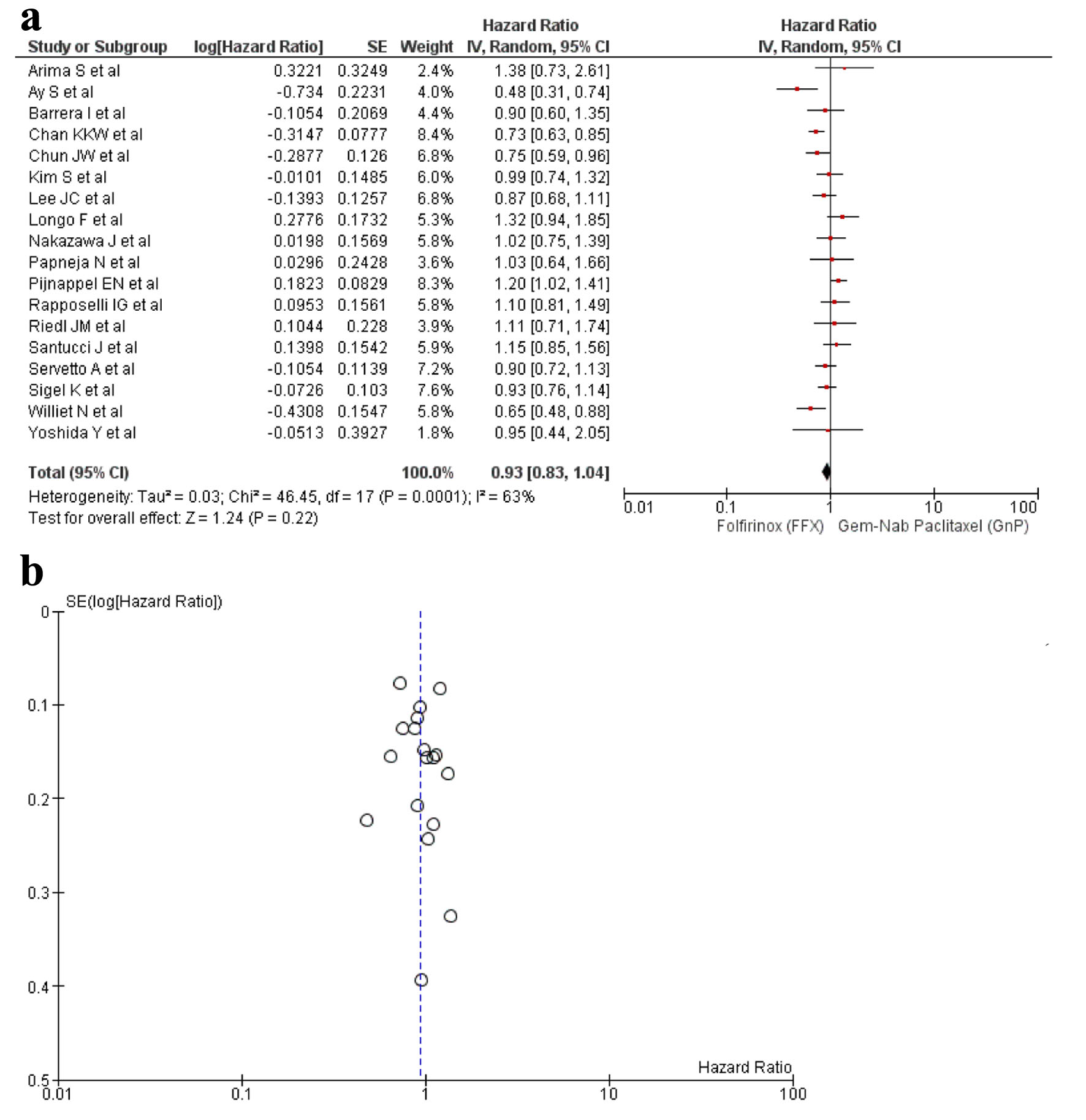 Click for large image | Figure 2. (a) Forest plot of OS (HR: 0.93; 95% CI: 0.83 - 1.04; P = 0.0001). (b) Funnel plot of OS. HR: hazard ratio; CI: confidence interval; OS: overall survival. |
PFS
A total of 13 studies [16-22, 24, 25, 27, 29, 32, 34] reported PFS and a significant difference was observed between the use of FFX and GnP as the first-line treatment for PC (HR: 0.88, 95% CI: 0.81 - 0.97; P < 0.0001) (Fig. 3a, b). Sensitivity analysis was conducted and removal of studies yielded a non-significant result (HR: 0.93, 95% CI: 0.85 - 1.02; P = 0.04) (Supplementary Material 7, www.wjon.org).
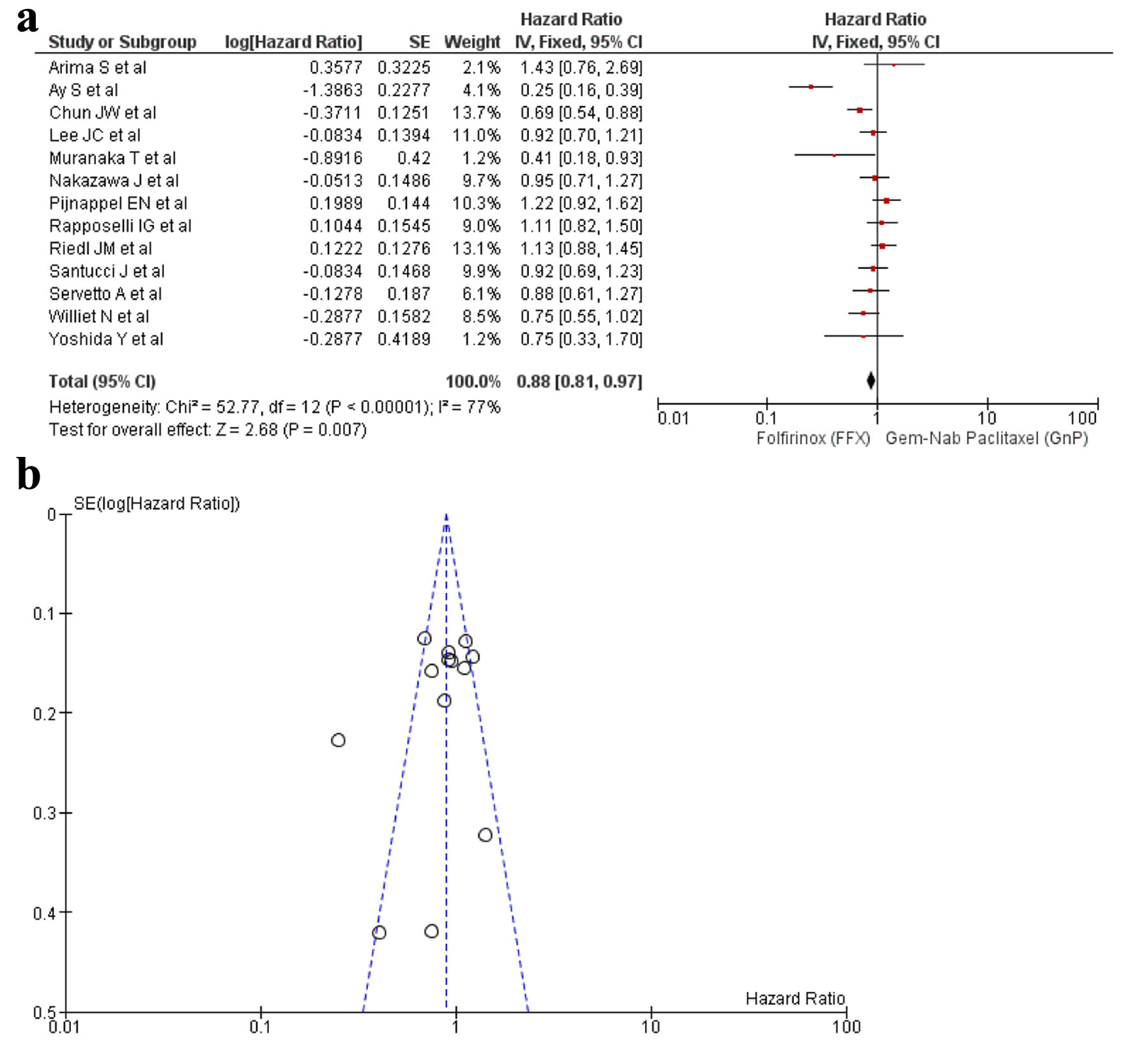 Click for large image | Figure 3. (a) Forest plot of PFS (HR: 0.88; 95% CI: 0.81 - 0.97; P < 0.0001). (b) Funnel plot of PFS. PFS: progression-free survival; HR: hazard ratio; CI: confidence interval. |
ORR
Seven studies [16, 20, 25, 27, 32, 34, 35] reported ORR with a total of 1,274 patients. There were 556 patients in the FFX arm, while 718 patients in the GnP arm. No statistical difference was observed between the use of two drugs (OR: 0.90; 95% CI: 0.64 - 1.27; P = 0.15) (Fig. 4a, b).
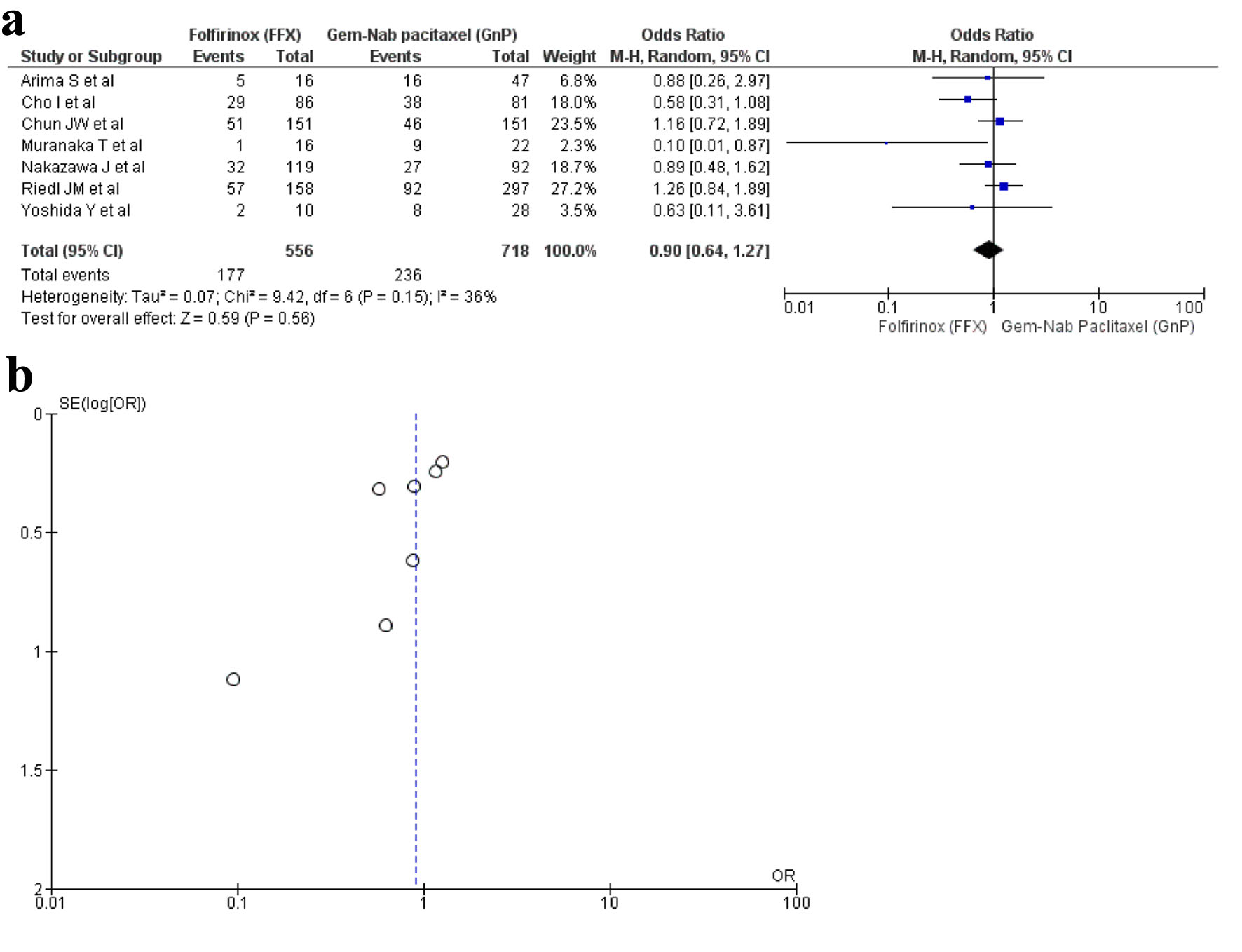 Click for large image | Figure 4. (a) Forest plot of objective response rate (OR: 0.90; 95% CI: 0.64 - 1.27; P = 0.15). (b) Funnel plot of objective response rate. OR: odds ratio; CI: confidence interval. |
Hematological outcomes
Anemia
Ten studies [16, 17, 19, 22, 28-30, 33, 34, 36] reported anemia as an AE with a total of 2,379 patients. There were 1,136 in the FFX arm, while 1,243 in the GnP arm. A statistical difference was observed as anemia was observed more in the FFX arm as compared to the GnP group (OR: 0.70, 95% CI: 0.51 - 0.98; P = 0.10) (Fig. 5a, b).
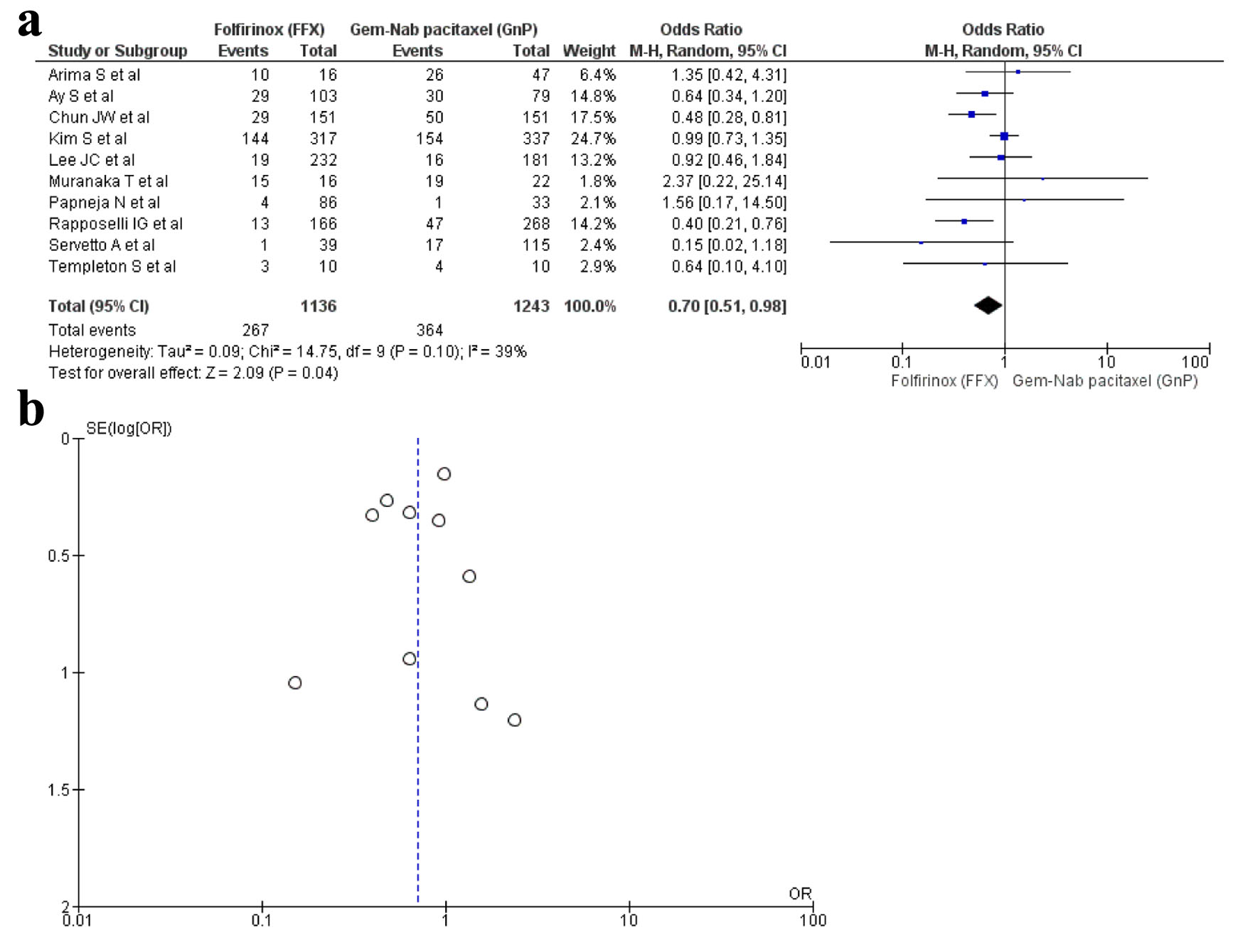 Click for large image | Figure 5. (a) Forest plot of anemia (OR: 0.70; 95% CI: 0.51 - 0.98; P = 0.10). (b) Funnel plot of anemia. OR: odds ratio; CI: confidence interval. |
Neutropenia
A total of 13 studies [16, 17, 19, 20, 22, 23, 28-30, 32-34, 36] with 3,713 patients reported neutropenia with 1,764 patients in the FFX group while 1,949 patients in the GnP group. The results were non-significant; hence, there was no difference seen between the two regimens in causing neutropenia (OR: 1.10; 95% CI: 0.92 - 1.31; P = 0.33) (Fig. 6a, b).
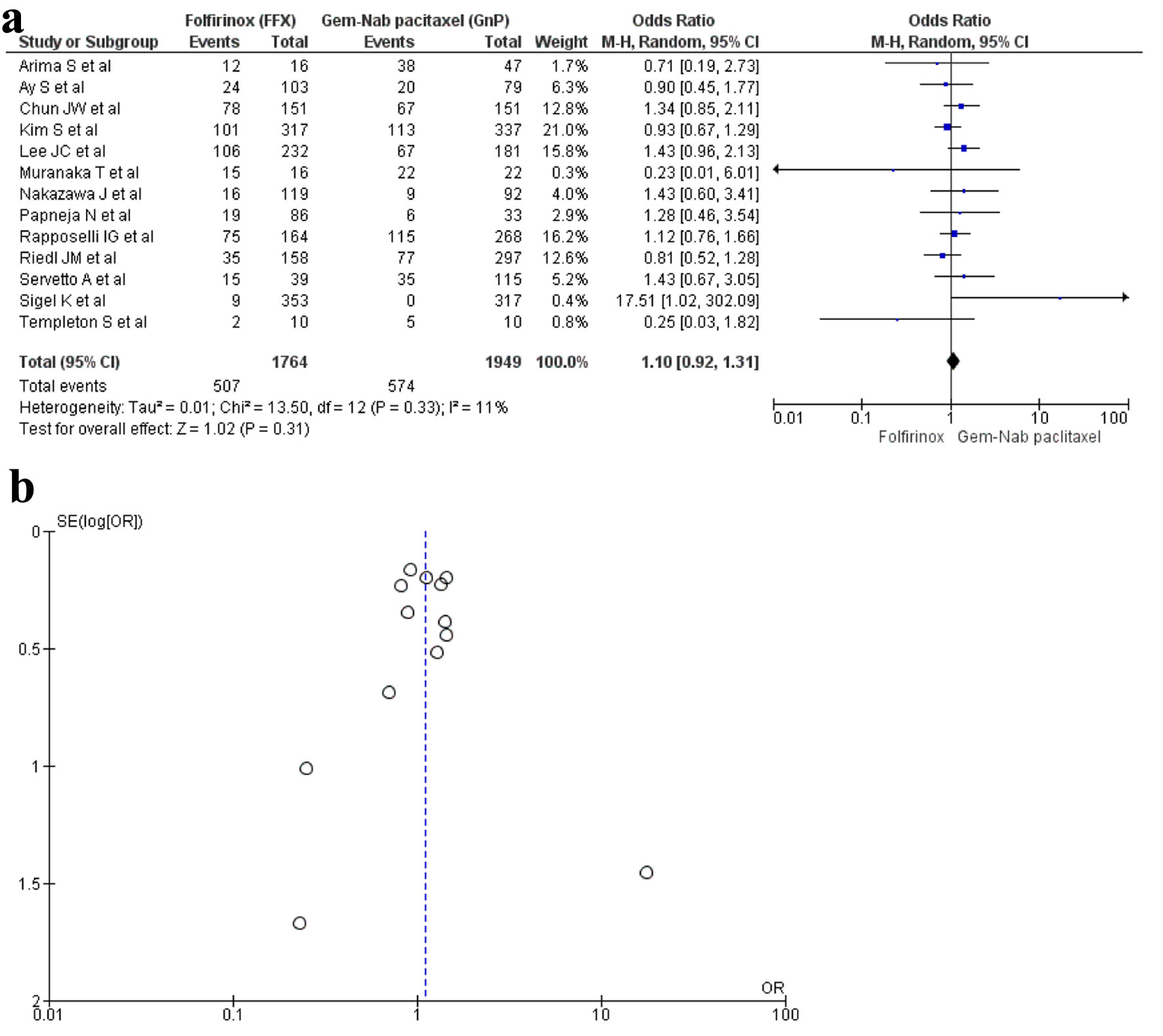 Click for large image | Figure 6. (a) Forest plot of neutropenia (OR: 1.10; 95% CI: 0.92 - 1.31; P = 0.33). (b) Funnel plot of neutropenia. OR: odds ratio; CI: confidence interval. |
Thrombocytopenia
Ten studies [16, 17, 19, 22, 28-30, 32, 34, 36] reported thrombocytopenia with a total of 2,369 patients with 1,126 in FFX group in contrast to 1,243 in the GnP group. There was no statistical significance seen in the two drug choices that caused thrombocytopenia (OR: 0.83; 95% CI: 0.60 - 1.13; P = 0.23) (Fig. 7a, b).
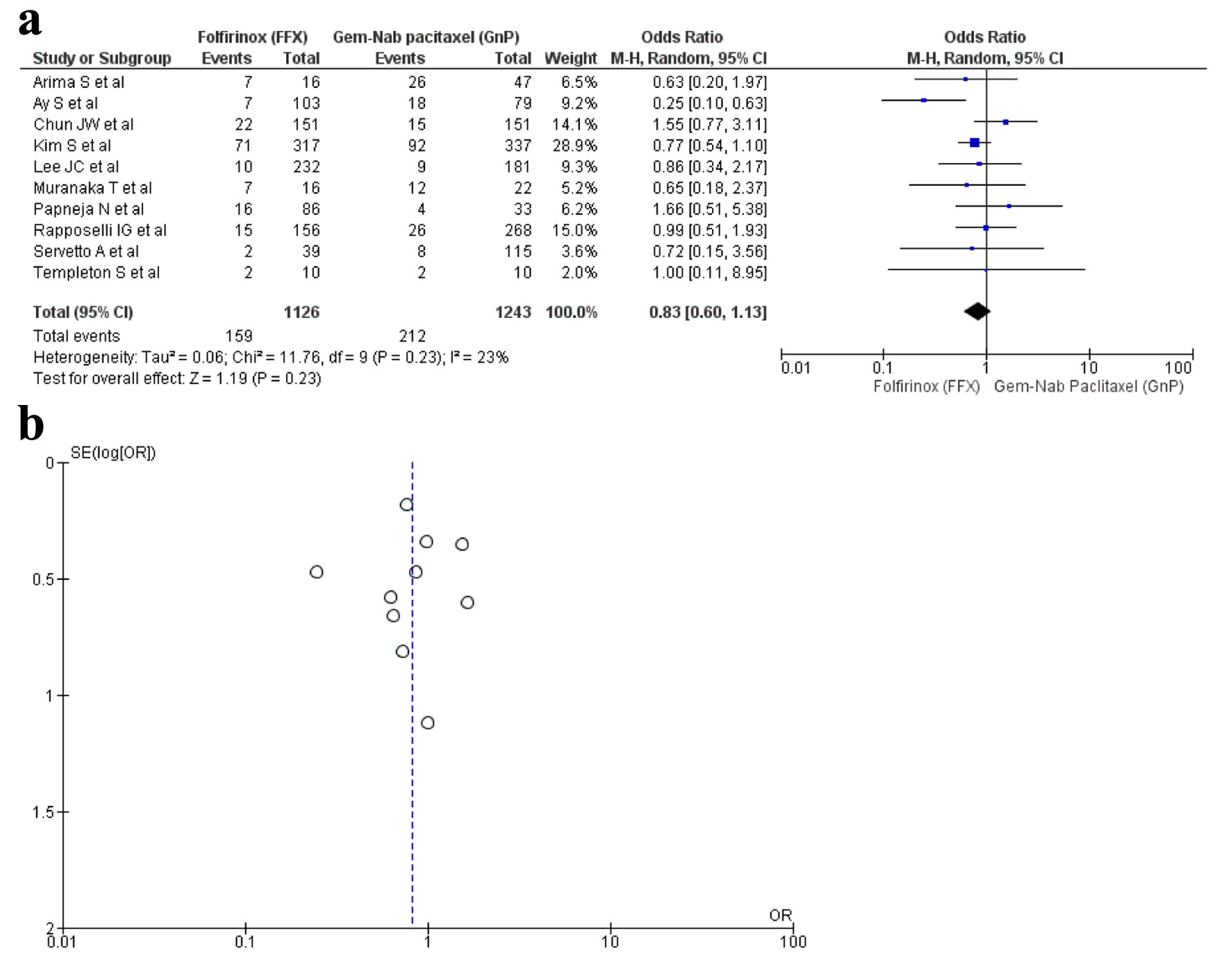 Click for large image | Figure 7. (a) Forest plot of thrombocytopenia (OR: 0.83; 95% CI: 0.60 - 1.13; P = 0.23). (b) Funnel plot of thrombocytopenia. |
Non-hematological outcomes
Diarrhea
A total of 12 studies [16, 17, 19, 20, 22, 23, 29, 30, 32-34, 36] reported diarrhea as an AE with a total of 3,421 patients. A total of 1,616 patients were recorded in the FFX group and 1,807 patients in the GnP group. The results were significant as diarrhea was observed more in the GnP group when compared to FFX (OR: 1.96; 95% CI: 1.22 - 3.15; P = 0.001) (Fig. 8a, b).
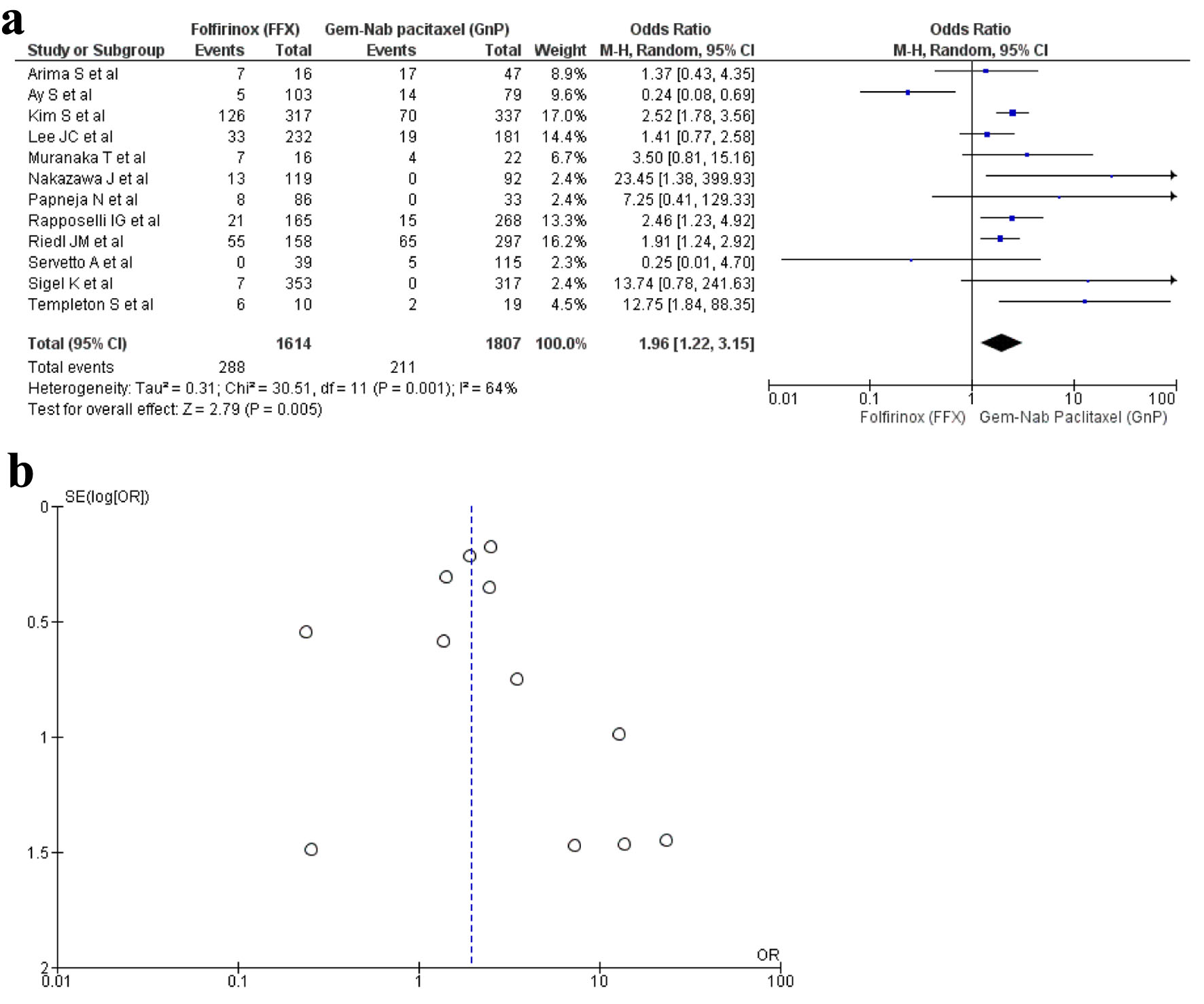 Click for large image | Figure 8. (a) Forest plot of diarrhea (OR: 1.96; 95% CI: 1.22 - 3.15; P = 0.001). (b) Funnel plot of diarrhea. OR: odds ratio; CI: confidence interval. |
| Discussion | ▴Top |
Our meta-analysis of more than 7,000 patients evaluated the efficacy and safety of two drug regimens, FFX and GnP, as the first-line treatment for advanced and metastatic PC (Fig. 1). As summarized in numerous trails and studies, our analysis also showed no statistical difference in increasing the OS time [10, 16, 19-23, 25, 29, 37]. Although previous meta-analyses have shown no significant difference in the survival outcomes of these drugs, this is the first study which showed a statistically significant increase in the PFS with use of FFX as the first-line treatment. Safety was evaluated in various trials and studies along with efficacy. Differences were found in safety profile of using these drugs with FFX being more associated with anemia while diarrhea occurred more in patients who used GnP.
Our study evaluated the OS time and the results were consistent with the previous meta-analysis and studies that used FFX and GnP as first-line treatments for PC, although five studies have shown that FFX is likely to be associated with a longer OS when compared to GnP [17, 18, 27, 28, 38]. There was no statistical difference found with a longer PFS with the use of FFX or GnP but pooling of studies and a larger number of participants from previous meta-analysis showed that FFX was associated with a longer PFS in contrast to GnP [10]. Safety profiles have differed between the two regimens with a difference of results in multiple studies. Two studies suggested the use of GnP as the first-line treatment due to its reduced cost and AEs while one study suggested that FFX has better survival and safety outcomes hence it should be the drug of choice for PC [29, 33, 35]. Furthermore, our study showed a statistical difference with anemia being more likely to be associated with FFX while diarrhea being associated with GnP. Our results differed from another study which suggested that FFX was more associated with diarrhea while FFX was more likely to cause anemia [19]. ORR was found to be statistically significant in the previous meta-analysis but pooling individual studies showed no statistical difference in ORR between the two regimens [10].
Previous studies have shown to differ in their choice of drug to be used as a first-line treatment in PC. Although studies have concluded that using FFX is likely to be associated with increased OS and PFS but due to a smaller testing sample size, a firm relation is yet to be established. As FFX is a combination of drugs, a larger sample population can shift the tables towards the use of FFX as the first-line drug. Irinotecan, a drug in the combination, has shown to exert its specific and synergistic effects on metastatic PC along with 5-FU and calcium folinate [39-42]. Furthermore, a trial concluded that oxaliplatin produced its clinical effects on PC only when it was used in combination with 5-FU [43]. Lastly a clinical trial summarized that use of irinotecan, oxaliplatin, 5-FU, and calcium leucovorin together can produce clinical benefits in patients with PC [44]. On the other hand, toxicities of any drug cannot be ignored. FFX is associated with hematological toxicities due to the mechanism of action of drug itself. Previous studies have shown that use of oxaliplatin as an anticancer agent has caused bone marrow suppression and blood system toxicities such as thrombocytopenia [45]. In contrast GnP is considered as a much safer option as summarized by two studies, hence GnP is considered to be safer when it comes to hematological safety outcomes [29, 35].
Considering the implications and cause of heterogeneity, we should keep in mind that the selection of these drugs to be used in patients as first-line treatment for PC depends on several factors that ultimately contribute to its efficacy. Such factors include socio-economic status, ethnicity, ECOG performance score, age, baseline characteristics and comorbidities. In MPACT trial, use of GnP showed a longer survival in Asian subgroup when compared to the western subgroup [46]. In addition, a meta-analysis showed that Asian subgroup had more intense AEs as compared to the western subgroup [47]. Hence it can be concluded that non-uniformity in the ethnicity of patients could be a possible factor for heterogeneity.
In our study, there were some limitations that need to be addressed. Randomization of patients based on demographic factors, ethnicity, baseline characteristics, age and comorbidities could not be possible as retrospective studies were only included in this analysis. Furthermore, due to a relatively small sample size, the results might have been overestimated leading to biasness. The patients differed in their baseline characteristics owing to an overall biasness and heterogeneity when recording up AEs. Lastly due to incomplete follow-up, potential biasness in the calculation of PFS and OS could not be avoided. Nevertheless, we analyzed the efficacy and safety of two regimens on a larger sample size from the previous meta-analysis and tried our best to include propensity-matched data to eradicate any biasness and heterogeneity.
| Conclusion | ▴Top |
FFX is more associated with prolonging PFS, while no difference is seen in OS of patients that used FFX or GnP as the first-line drug. AEs or toxicities are a key factor to determine the use of any drug for a particular disease, hence with FFX being more associated with hematological outcomes while GnP being more likely to be associated with non-hematological outcomes, there is a dire need for future trials to be conducted on a large sample size population to determine the drug of choice to be used as first-line treatment for PC.
| Supplementary Material | ▴Top |
Suppl 1. PubMed - detailed search strategy.
Suppl 2. PRISMA flow diagram for retrieval of articles.
Suppl 3. Graphical representation of the study in a systematic way.
Suppl 4. Quality assessment of articles performed through QUADS-2 tool.
Suppl 5. (A) Distribution of tumor locations in pancreatic head, body, and tail. Series 1 and 2 show the prevalence of tumors within different pancreas regions. (B) Distribution of tumor locations in pancreatic head, body, and tail. Series 1 and 2 depict the spatial distribution and prevalence of tumors within different pancreas regions.
Suppl 6. (A) Forest plot of overall survival (OS) sensitivity analysis (HR: 0.91, 95% CI: 0.81 - 1.01; P = 0.005). (B) Funnel plot of OS sensitivity analysis.
Suppl 7. (A) Forest plot of progression-free survival (PFS) sensitivity analysis (HR: 0.93, 95% CI: 0.85 - 1.02; P = 0.04). (B) Funnel plot of PFS sensitivity analysis.
Acknowledgments
None to declare.
Financial Disclosure
This study required no funding.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author Contributions
Nooraldin Merza, Sabeeh Khawar Farooqui, Sophia Haroon Dar, and Tony Varughese: manuscript writing, an overview of the previously published works on a topic. Rehmat Ullah Awan, Lamaan Qureshi, and Saad Ali Ansari: database extraction, results, and statistics. Saad Ali Ansari and Jamie Mcilvaine: manuscript preparation and editing. Hadi Qureshi: data collection, manuscript preparation, and editing content. Ali Nawras, Yaseen Alastal, and Mona Hassan: drafting and approval of final version, manuscript review, critical revision of the manuscript for important intellectual content.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
Abbreviations
FFX: Folfirinox; GnP: gemcitabine + nab-paclitaxel; HRs: hazard ratios; OR: odds ratio; ORR: objective response rate; OS: overall survival; PC: pancreatic cancer; PFS: progression-free survival
| References | ▴Top |
- Kamisawa T, Wood LD, Itoi T, Takaori K. Pancreatic cancer. Lancet. 2016;388(10039):73-85.
doi pubmed - Jung KW, Won YJ, Oh CM, Kong HJ, Lee DH, Lee KH. Prediction of cancer incidence and mortality in Korea, 2017. Cancer Res Treat. 2017;49(2):306-312.
doi pubmed pmc - Gawron AJ, Gapstur SM, Fought AJ, Talamonti MS, Skinner HG. Sociodemographic and tumor characteristics associated with pancreatic cancer surgery in the United States. J Surg Oncol. 2008;97(7):578-582.
doi pubmed - Baxter NN, Whitson BA, Tuttle TM. Trends in the treatment and outcome of pancreatic cancer in the United States. Ann Surg Oncol. 2007;14(4):1320-1326.
doi pubmed - Sohal DP, Mangu PB, Khorana AA, Shah MA, Philip PA, O'Reilly EM, Uronis HE, et al. Metastatic Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2016;34(23):2784-2796.
doi pubmed pmc - Burris HA, 3rd, Moore MJ, Andersen J, Green MR, Rothenberg ML, Modiano MR, Cripps MC, et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J Clin Oncol. 1997;15(6):2403-2413.
doi pubmed - Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369(18):1691-1703.
doi pubmed pmc - Herrmann R, Bodoky G, Ruhstaller T, Glimelius B, Bajetta E, Schuller J, Saletti P, et al. Gemcitabine plus capecitabine compared with gemcitabine alone in advanced pancreatic cancer: a randomized, multicenter, phase III trial of the Swiss Group for Clinical Cancer Research and the Central European Cooperative Oncology Group. J Clin Oncol. 2007;25(16):2212-2217.
doi pubmed - Conroy T, Desseigne F, Ychou M, Bouche O, Guimbaud R, Becouarn Y, Adenis A, et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N Engl J Med. 2011;364(19):1817-1825.
doi pubmed - Chen J, Hua Q, Wang H, Zhang D, Zhao L, Yu D, Pi G, et al. Meta-analysis and indirect treatment comparison of modified FOLFIRINOX and gemcitabine plus nab-paclitaxel as first-line chemotherapy in advanced pancreatic cancer. BMC Cancer. 2021;21(1):853.
doi pubmed pmc - Kang J, Hwang I, Yoo C, Kim KP, Jeong JH, Chang HM, Lee SS, et al. Nab-paclitaxel plus gemcitabine versus FOLFIRINOX as the first-line chemotherapy for patients with metastatic pancreatic cancer: retrospective analysis. Invest New Drugs. 2018;36(4):732-741.
doi pubmed - Peixoto RD, Ho M, Renouf DJ, Lim HJ, Gill S, Ruan JY, Cheung WY. Eligibility of metastatic pancreatic cancer patients for first-line palliative intent nab-paclitaxel plus gemcitabine versus FOLFIRINOX. Am J Clin Oncol. 2017;40(5):507-511.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
doi pubmed pmc - http://www.clinicaltrials.gov
- http://www.clinicaltrialresults.org
- Arima S, Kawahira M, Shimokawa M, Ido A, Koga F, Ueda Y, Nakazawa J, et al. Gemcitabine plus nab-paclitaxel versus FOLFIRINOX in locally advanced, unresectable pancreatic cancer: a multicenter observational study (NAPOLEON Study). Pancreas. 2021;50(7):957-964.
doi pubmed - Ay S, Atci MM, Arikan R, Dulgar O, Ozyukseler DT, Paksoy N, Dogan I, et al. FOLFIRINOX versus gemcitabine plus nab-paclitaxel as the first-line chemotherapy in metastatic pancreatic cancer. J Chemother. 2022;34(7):465-471.
doi pubmed - Pijnappel EN, Dijksterhuis WPM, van der Geest LG, de Vos-Geelen J, de Groot JWB, Homs MYV, Creemers GJ, et al. First- and second-line palliative systemic treatment outcomes in a real-world metastatic pancreatic cancer cohort. J Natl Compr Canc Netw. 2021;20(5):443-450.e443.
doi pubmed - Rapposelli IG, Casadei-Gardini A, Vivaldi C, Bartolini G, Bernardini L, Passardi A, Frassineti GL, et al. Equivalent efficacy but different safety profiles of gemcitabine plus nab-paclitaxel and FOLFIRINOX in metastatic pancreatic cancer. Biomolecules. 2021;11(6):780.
doi pubmed pmc - Riedl JM, Posch F, Horvath L, Gantschnigg A, Renneberg F, Schwarzenbacher E, Moik F, et al. Gemcitabine/nab-Paclitaxel versus FOLFIRINOX for palliative first-line treatment of advanced pancreatic cancer: A propensity score analysis. Eur J Cancer. 2021;151:3-13.
doi pubmed - Santucci J, Tacey M, Thomson B, Michael M, Wong R, Shapiro J, Jennens R, et al. Impact of first-line FOLFIRINOX versus Gemcitabine/Nab-Paclitaxel chemotherapy on survival in advanced pancreatic cancer: Evidence from the prospective international multicentre PURPLE pancreatic cancer registry. Eur J Cancer. 2022;174:102-112.
doi pubmed - Servetto A, Santaniello A, Napolitano F, Foschini F, Marciano R, Cascetta P, Amato AR, et al. FOLFIRINOX or nab-paclitaxel plus gemcitabine in metastatic pancreatic adenocarcinoma: an observational study. Future Oncol. 2022;18(21):2643-2653.
doi pubmed - Sigel K, Zhou M, Park YA, Mutetwa T, Nadkarni G, Yeh C, Polak P, et al. Gemcitabine plus nab-paclitaxel versus FOLFIRINOX for unresected pancreatic cancer: Comparative effectiveness and evaluation of tumor growth in Veterans. Semin Oncol. 2021;48(1):69-75.
doi pubmed pmc - Williet N, Saint A, Pointet AL, Tougeron D, Pernot S, Pozet A, Bechade D, et al. Folfirinox versus gemcitabine/nab-paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study. Therap Adv Gastroenterol. 2019;12:1756284819878660.
doi pubmed pmc - Yoshida Y, Kobayashi S, Ueno M, Morizane C, Tsuji K, Maruki Y, Mori K, et al. Efficacy of chemotherapy for patients with metastatic or recurrent pancreatic adenosquamous carcinoma: A multicenter retrospective analysis. Pancreatology. 2022;22(8):1159-1166.
doi pubmed - Barrera I, Ranger J, Roofigari N, Dalfen R, Batist G, Kavan P. Treatment sequencing in MPC, insights from a 3° care center. J Clin Oncol. 2019;37(4_suppl):400.
- Chan KKW, Guo H, Cheng S, Beca JM, Redmond-Misner R, Isaranuwatchai W, Qiao L, et al. Real-world outcomes of FOLFIRINOX vs gemcitabine and nab-paclitaxel in advanced pancreatic cancer: A population-based propensity score-weighted analysis. Cancer Med. 2020;9(1):160-169.
doi pubmed pmc - Chun JW, Lee SH, Kim JS, Park N, Huh G, Cho IR, Paik WH, et al. Comparison between FOLFIRINOX and gemcitabine plus nab-paclitaxel including sequential treatment for metastatic pancreatic cancer: a propensity score matching approach. BMC Cancer. 2021;21(1):537.
doi pubmed pmc - Kim S, Signorovitch JE, Yang H, Patterson-Lomba O, Xiang CQ, Ung B, Parisi M, et al. Comparative effectiveness of nab-paclitaxel plus gemcitabine vs FOLFIRINOX in metastatic pancreatic cancer: a retrospective nationwide chart review in the United States. Adv Ther. 2018;35(10):1564-1577.
doi pubmed pmc - Lee JC, Woo SM, Shin DW, Kim J, Yang SY, Kim MJ, Kim JW, et al. Comparison of FOLFIRINOX and gemcitabine plus nab-paclitaxel for treatment of metastatic pancreatic cancer: using Korean pancreatic cancer (K-PaC) registry. Am J Clin Oncol. 2020;43(9):654-659.
doi pubmed - Longo Munoz F, Castillo Trujillo O, Serrano Domingo J, Martin Huertas R, Corral de la Fuente E, San Juan A, et al. FOLFIRINOX versus Nab-paclitaxel plus gemcitabine in the first-line chemotherapy for patients with advanced pancreatic ductal adenocarcinoma: a multivariate analysis of prognostic factors in a national cohort (Comunica-TTD working group). Ann Oncol. 2019;30:iv92-iv93.
- Nakazawa J, Otsuka T, Shimokawa M, Koga F, Ueda Y, Otsu S, et al. A multicenter retrospective study of gemcitabine plus nab-paclitaxel or FOLFIRINOX in metastatic pancreatic cancer: NAPOLEON study. Ann Oncol. 2019;30:iv17-iv18.
- Papneja N, Zaidi A, Chalchal H, Moser M, Tan K, Olson C, Haider K, et al. Comparisons of outcomes of real-world patients with advanced pancreatic cancer treated with FOLFIRINOX versus gemcitabine and nab-paclitaxel: a population-based cohort study. Pancreas. 2019;48(7):920-926.
doi pubmed - Muranaka T, Kuwatani M, Komatsu Y, Sawada K, Nakatsumi H, Kawamoto Y, Yuki S, et al. Comparison of efficacy and toxicity of FOLFIRINOX and gemcitabine with nab-paclitaxel in unresectable pancreatic cancer. J Gastrointest Oncol. 2017;8(3):566-571.
doi pubmed pmc - Cho I, Kang H, Jo J, Lee H, Chung M, Park J, et al. FOLFIRINOX versus gemcitabine plus nab-paclitaxel for treatment of metastatic pancreatic cancer: a single-center cohort study. Ann Oncol. 2018;29:v45.
- Templeton S, Moser M, Wall C, Shaw J, Chalchal H, Luo Y, Zaidi A, et al. Outcomes of patients with borderline resectable pancreatic cancer treated with combination chemotherapy. J Gastrointest Cancer. 2021;52(2):529-535.
doi pubmed - Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, Paranjpe I, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32(1):151-160.
doi pubmed pmc - Zhang B, Zhou F, Hong J, Ng DM, Yang T, Zhou X, Jin J, et al. The role of FOLFIRINOX in metastatic pancreatic cancer: a meta-analysis. World J Surg Oncol. 2021;19(1):182.
doi pubmed pmc - Azrak RG, Cao S, Slocum HK, Toth K, Durrani FA, Yin MB, Pendyala L, et al. Therapeutic synergy between irinotecan and 5-fluorouracil against human tumor xenografts. Clin Cancer Res. 2004;10(3):1121-1129.
doi pubmed - Mans DR, Grivicich I, Peters GJ, Schwartsmann G. Sequence-dependent growth inhibition and DNA damage formation by the irinotecan-5-fluorouracil combination in human colon carcinoma cell lines. Eur J Cancer. 1999;35(13):1851-1861.
doi pubmed - Mullany S, Svingen PA, Kaufmann SH, Erlichman C. Effect of adding the topoisomerase I poison 7-ethyl-10-hydroxycamptothecin (SN-38) to 5-fluorouracil and folinic acid in HCT-8 cells: elevated dTTP pools and enhanced cytotoxicity. Cancer Chemother Pharmacol. 1998;42(5):391-399.
doi pubmed - Pavillard V, Formento P, Rostagno P, Formento JL, Fischel JL, Francoual M, Etienne MC, et al. Combination of irinotecan (CPT11) and 5-fluorouracil with an analysis of cellular determinants of drug activity. Biochem Pharmacol. 1998;56(10):1315-1322.
doi pubmed - Ducreux M, Mitry E, Ould-Kaci M, Boige V, Seitz JF, Bugat R, Breau JL, et al. Randomized phase II study evaluating oxaliplatin alone, oxaliplatin combined with infusional 5-FU, and infusional 5-FU alone in advanced pancreatic carcinoma patients. Ann Oncol. 2004;15(3):467-473.
doi pubmed - Conroy T, Paillot B, Francois E, Bugat R, Jacob JH, Stein U, Nasca S, et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer—a Groupe Tumeurs Digestives of the Federation Nationale des Centres de Lutte Contre le Cancer study. J Clin Oncol. 2005;23(6):1228-1236.
doi pubmed - Jardim DL, Rodrigues CA, Novis YAS, Rocha VG, Hoff PM. Oxaliplatin-related thrombocytopenia. Ann Oncol. 2012;23(8):1937-1942.
doi pubmed - Goldstein D, Von Hoff DD, Moore M, Greeno E, Tortora G, Ramanathan RK, Macarulla T, et al. Development of peripheral neuropathy and its association with survival during treatment with nab-paclitaxel plus gemcitabine for patients with metastatic adenocarcinoma of the pancreas: A subset analysis from a randomised phase III trial (MPACT). Eur J Cancer. 2016;52:85-91.
doi pubmed - Lee YS, Lee JC, Kim JH, Kim J, Hwang JH. Pharmacoethnicity of FOLFIRINOX versus gemcitabine plus nab-paclitaxel in metastatic pancreatic cancer: a systematic review and meta-analysis. Sci Rep. 2021;11(1):20152.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


