| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 15, Number 3, June 2024, pages 355-371
Intercontinental Comparison of Immunohistochemical Subtypes Among Individuals With Breast Cancer in South-East Asia and South America: A Scoping Systematic Review and Meta-Analysis of Observational Studies
Dedy Hermansyaha, f , Naufal Nandita Firstyb, c
, Ruth Hasian Nami Siagianb, d
, Najwa Nandita Dwindae
aDivision of Surgical Oncology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
bGraduate Program in Medicine, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
cPutri Hijau Level II Military Hospital, Medan, Indonesia
dDatu Sanggul Rantau Public Hospital, Tapin, Indonesia
eUndergraduate Program in Public Health, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
fCorresponding Author: Dedy Hermansyah, Division of Surgical Oncology, Department of Surgery, Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia
Manuscript submitted January 29, 2024, accepted April 11, 2024, published online May 7, 2024
Short title: BC’s Subtypes in South-East Asia and South America
doi: https://doi.org/10.14740/wjon1788
| Abstract | ▴Top |
Background: Breast cancer (BC) remains a significant global concern, particularly among developing countries in South-East Asia (SEA) and South America (SA). The socioeconomic burdens of oncologic care in those countries were often originated from limited accessibility on attainable therapeutic options and reliability on identifying essential information of cancer cells, i.e., immunohistochemical (IHC) subtyping to determine suitable approaches. The triple-negative breast cancer (TNBC) is among the most aggressive category in breast malignancy, therefore, requiring more specific molecular pathway blocking to exhaust the cells. However, large-scale epidemiological investigation on its rate among BC remains unavailable to date. This study aimed to describe the prevalence of TNBC in the SEA and SA continents since it may guide the future direction of oncologic research and trials.
Methods: This review focuses on observational studies from the SEA and SA continents from the last decade. Each study represents its country or cities, period of observation, population size, and the TNBC-BC rate as the main outcomes. Therefore, we may also limit the reporting bias originated from same-patient data on the specific occasions. The analysis will be derived to SEA-SA comparison, plus SEA/SA-specific session as processed in Comprehensive Meta-Analysis (CMA) version 3.0. The statistical analysis will be performed in random effects model (REM) within 95% confidence interval (CI).
Results: From 46 studies included in the final analysis with a total enlisted population of 34,346 unique individuals with BC, the TNBC rate was higher in the SEA compared to the SA region (19.3% vs. 15.7%; P < 0.05 in 95% CI), with the highest prevalence observed in Vietnam (22.4%) and Peru (17.8%), if it was restricted on countries with two or more studies. Interestingly, both Laos and Argentina possessed significant differences compared to other countries within their respective continents, with the highest and lowest TNBC rates (P < 0.05).
Conclusions: The IHC characteristics in SEA differ from those in the SA continent as mainly represented by TNBC prevalence, possibly shaping the course of future trials in the respective region based on IHC expressivity status.
Keywords: Breast cancer; Epidemiology; Immunohistochemical subtype; South America; South-East Asia
| Introduction | ▴Top |
The importance of planning a grand design for better cancer care is progressively escalating every year, whether by identifying a potential molecular target, or by developing an excellent inter-professional collaboration, involving early cancer detection initiative to prevent costly treatment sessions. The tentative nature of oncology science is considered as the premier aspect in evidence-based medicine, as more progress reported, more study presented, and more individual involved in the community. Yet, we believe that capturing the culprit of a global-level issue may be a humble and simple idea, as straightforward as describing the epidemiological data based on descriptive investigations from parts of the globe. For instance, breast cancer (BC) possessed the highest incidence rate according to the GLOBOCAN 2020 report, with 11.7% of total malignancies worldwide and accountable for > 600,000 deaths annually. It even almost reaches a quarter percentage among females, leading to the higher demand of BC-related research, hence considerate framework arrangement for global effort on cancer groundwork should be based on the specified-demand from each region [1].
The triple-negative breast cancer (TNBC) can be viewed as the exemplary situation of the necessity to develop more population-based studies on aggressive subtypes, since it is predicted that the expression rate might differ among continents [2]. Generally, it was estimated to be 15-25% of all BC, and some race or ethnicity is observed to be at higher risk for not expressing both progesterone/estrogen receptor (PR/ER) and human epidermal growth factor-2 (HER-2) on its molecular profiling [3, 4]. Those missing links predetermined the initial treatment guideline, because the classical blockable targets are absent, and it will dictate the research direction to much more complex research involving more targeted treatment or personalized medicine.
To represent the urgency, the US National Library of Medicine on the clinicaltrials.gov stated that from 812 active trials on TNBC globally, only 4.1% and 4.6% of the studies originated from the South-East Asia (SEA) and South America (SA; or Latin America); respectively, per December 1, 2023. Interestingly, the USA holds at least 60% of the total trials, followed by the Europe (28.6%) and China (23.9%) [5]. Supporting the premise, a total of 339,833,728 and 250,584,187 females in both SEA and SA according to The World Bank data in 2022 are a plethora of potential cases, compared to “roughly” 168,266,219 from USA with significantly higher trial rates [6]. Therefore, there was a relative imbalance of trials-population rate on those continents, and it will be intriguing to deliver the immunohistochemistry (IHC) status from such populated regions of SEA and SA, since it may prove the necessity to include the continents in future investigations. Both continents also share many similarities, e.g., socioeconomic status which is dominated by developing nations, overall landscape, cultural aspects, etc., which creates a suitable soil to be compared from the quantitative-epidemiological perspectives. Geographically, both continents were separated by vast Pacific Ocean or the Africa continent, located on the different side of the globe quite literally [7, 8]. Some evidence also suggested a series of genetic linkages of the populations tracked back to early human migration, though thousand years of different influence in the regions may alter the varieties profoundly [9].
For that reason, this study aimed to provide a comparison of the TNBC rate in both SEA and SA regions as a representative and triggering statistical evidence of the IHC distribution across the globe in such similar demographic natures. Therefore, it is expected to reach the responsible stakeholders on why it is also required to provide additional considerations in incorporating those populations into the upcoming TNBC trials.
| Materials and Methods | ▴Top |
Study design and protocol registration
To fulfill the main objective of this review, we designed the analysis to focus on the TNBC distribution as reported by each study based on the IHC evaluations toward histopathologically confirmed BC. We prepared the study in accordance with the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) protocol and performed double literature identification phases in the SEA and SA regions to improve the searching scope. Two authors (NNF and RHNS) introduced the screening strategy to the several scientific databases, including MEDLINE, ScienceDirect, and ProQuest by Boolean term method. They included vital keywords on MeSH such as “breast cancer”, “immunohistochemical”, “South-East Asia” or “South America”, and country-specific words (utilizing its name such as Indonesia, Brazil, Thailand, etc., in the search box based on the respective region). We anticipated to gather descriptive or cross-sectional studies at most (plus cohorts), since the base reasoning to conduct our study is rather simple yet important.
The intended publication date was limited to last decade report, which included the percentage of TNBC per total population (pooled BC population), and it is mandatory to confirm the BC subtypes based on IHC screening by credible anatomical pathology (or laboratory) facility. Registry- or laboratory-based studies will be excluded from the final analysis, considering its “data”-oriented evaluation rather than direct investigator-participant interaction. Moreover, we also exclude studies that had objectives differing from our statistical questions, e.g., restricting age-specified population, analyzing the socioeconomic impact of BC, etc. To prevent overlapping between investigations, we selected the study based on geographical identity (country, city, and centers), the observation period, and population size. From that point, studies with “smaller” population sizes during the similar time period, fewer centers included in a city/region, or even with shorter observation period, were also be excluded. The protocol of this review has been registered in PROSPERO: International Prospective Register of Systematic Reviews under issued identification (ID) of CRD42023466295 [10].
Risk-of-bias assessment and data extraction
The risk of bias will be performed in “adapted Newcastle-Ottawa Scale (NOS)” by three reviewers (NNF, RHNS, and NND) independently. This method was recently applied by Ribeiro et al in their systematic review with focus on cross-sectional studies, and we agreed to conduct the quality assessment by using the previously used tool for this issue [11]. Disagreement of each author’s judgement on the quality assessment will be resolved in separate internal discussion with the first author (DH). We interpreted the NOS assessment results based on the Agency for Healthcare Research and Quality (AHRQ) standards [12].
We identify key characteristics from each study such as first author’s last name, observation period, and included centers (either single- or multi-center investigations), city, country, and geographical location based on the continent (SEA or SA). The total sample size along with participants’ age description will also be included as supporting demographic identifiers. However, the main data extracted from the following studies will be oriented on TNBC distribution across specific period. To limit the tentative nature of patients reporting system between regions which represented by centers, we purposely restrict the reports by center-period identification, such as ABC General Hospital, Jakarta, Indonesia (July 2013 to December 2020). Therefore, the bias originated from the inclusion of the identical patient’s group should be subsequently reduced; though, it remains impractical to eliminate those questionable possibilities completely, considering the base design of our review, i.e., systematic review of observational studies.
Statistical analysis
The statistical presentation of our findings was processed in Comprehensive Meta-Analysis (CMA) version 3.0, by accessing the event rate data calculation through events-and-sample size ratio (ranging from 0.000 to 1.000) [13, 14]. We perform the analysis in three different phases by using random effects model (REM) to minimize the heterogeneity aspects, according to the respective SEA and SA region plus pooled investigation of both continents. In each continent or sub-analysis, the studies will be arranged according to its country (as the sub-group) from the highest to lowest event rate cumulatively. The mean event rate produced by each sub-group analysis will be dotted in red as presented in the forest plots. For the continent-representative TNBC rate, we will rely on the collective data provided on the pooled SEA and SA analysis rather than each continent-specific overall event rate estimation, hence the cumulative TNBC rates of either SEA or SA are presented on the pooled analysis.
The logit event rate will be provided as an additional consideration to predict the significance of difference between countries or continents, by observing the possible overlapping of lower- and upper-limit outcomes. In the event of no overlapping of the logit event rate, one may consider the difference might be statistically significant. However, we also presented the meta-regression model to demonstrate the two-sided P value estimation between the covariates (continent or countries), with the P value of < 0.05 should be considered as statistically significant. The meta-regression’s models will be constructed by linking the sub-covariates; hence we can estimate each inter-country P value based on the difference to the intercept covariate. This statistical presentation is aimed to furtherly enrich the perspective in assessing our review as a whole, albeit its main objective of issuing a descriptive meta-analysis of TNBC rate across SEA and SA continents.
| Results | ▴Top |
A total of 46 observational studies from both continents were included in the final analysis, with 28 investigations originating from the SEA region. Those studies encompassed 13,907 and 20,439 BC patients from SEA and SA regions, respectively; the total population of 34,346 individuals had been diagnostically validated through careful workup strategy plus mandatory IHC evaluations based on each center’s standard. The literatures selection process is depicted on Figure 1 in PRISMA flow diagram, adapted to our review’s specific modus operandi. The risk of bias and quality assessment of the included literatures were outlined here (Supplementary Material 1, www.wjon.org).
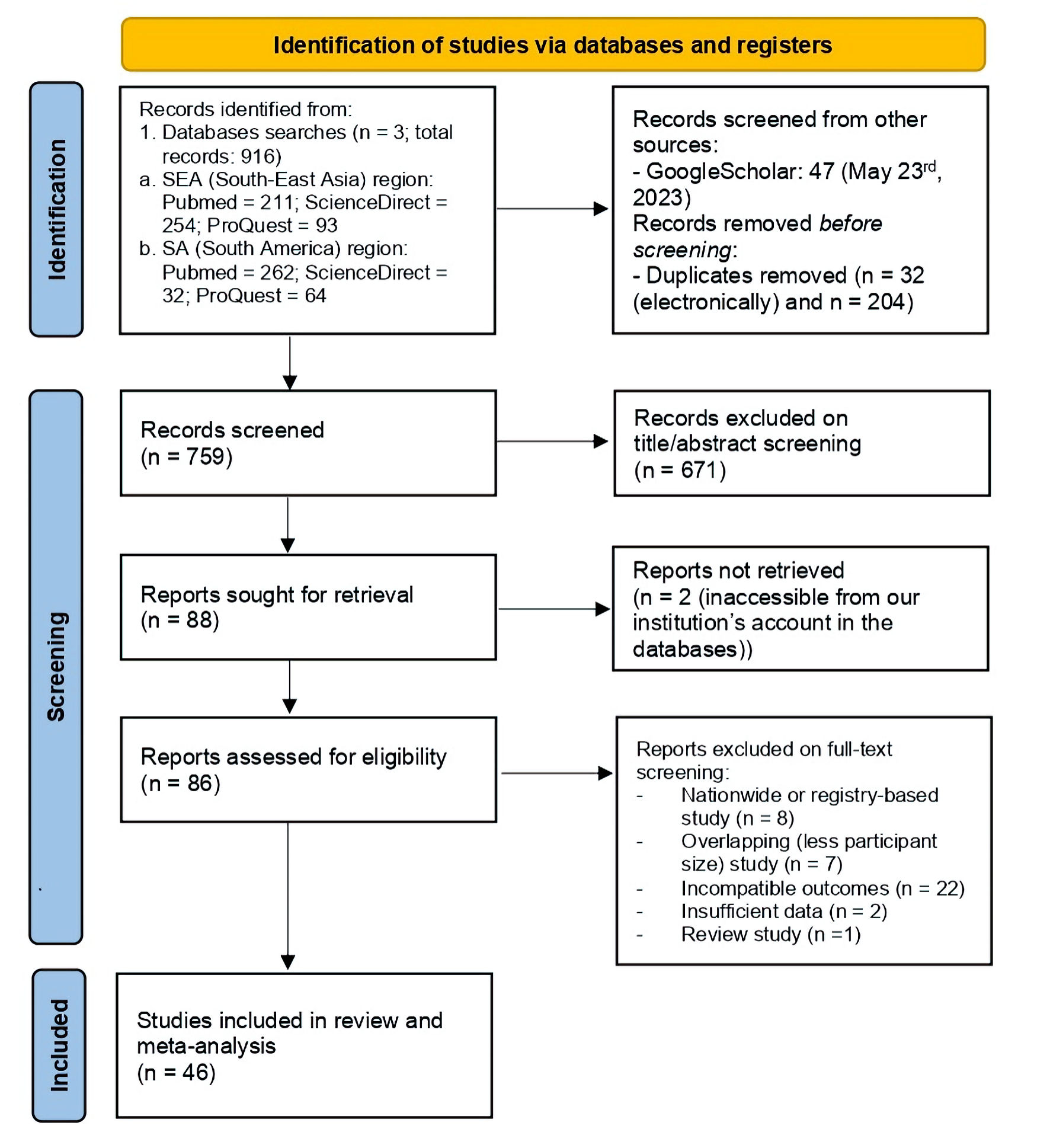 Click for large image | Figure 1. Study identification phases of this review in PRISMA flow diagram. PRISMA: the Preferred Reporting Items for Systematic Review and Meta-Analysis; SEA: South-East Asia; SA: South America. |
Based on the demographic characteristics (Table 1) [15-60], most of the studies from SEA and SA were based on Indonesia (2,788 patients) and Brazil (9,473 patients), respectively; though, Thailand holds the highest population number in SEA (6,685 patients). The SEA’s report included 20 cities (24 centers plus three smaller regions comprised of unmentioned number of hospitals); whereas the SA consisted of five nation scale reports covering unmentioned number of centers, and 13 province/cities with at least 13 different centers plus several private referral center for cancer care in a region (Table 1). The mean or median age of the populations are comparable in the fifth to sixth decade of life, albeit earlier diagnosis age is observable in SEA, with higher number of studies with the mean or median age < 50 years old.
 Click to view | Table 1. Geographical and Baseline Characteristics of the Included Studies |
Our collective analysis on both continents demonstrated that the overall TNBC rate is higher in SEA (19.3% (17.3-21.4%)), compared to the SA (15.7% (14.1-17.5%), with the cumulative assessment on the continents having 17.4% (16.1-18.8%) as the even rate, though it seems that the difference between those regions is not statistically significant (Fig. 2). Interestingly, several studies in the SEA region even recorded > 25.0% TNBC rate, conversely on the SA with all of the event rate estimations falling < 25.0% mark. The meta-regression model estimated the two-sided P value between the continents of 0.0085 (< 0.05), hence the difference between the regions is statistically significant.
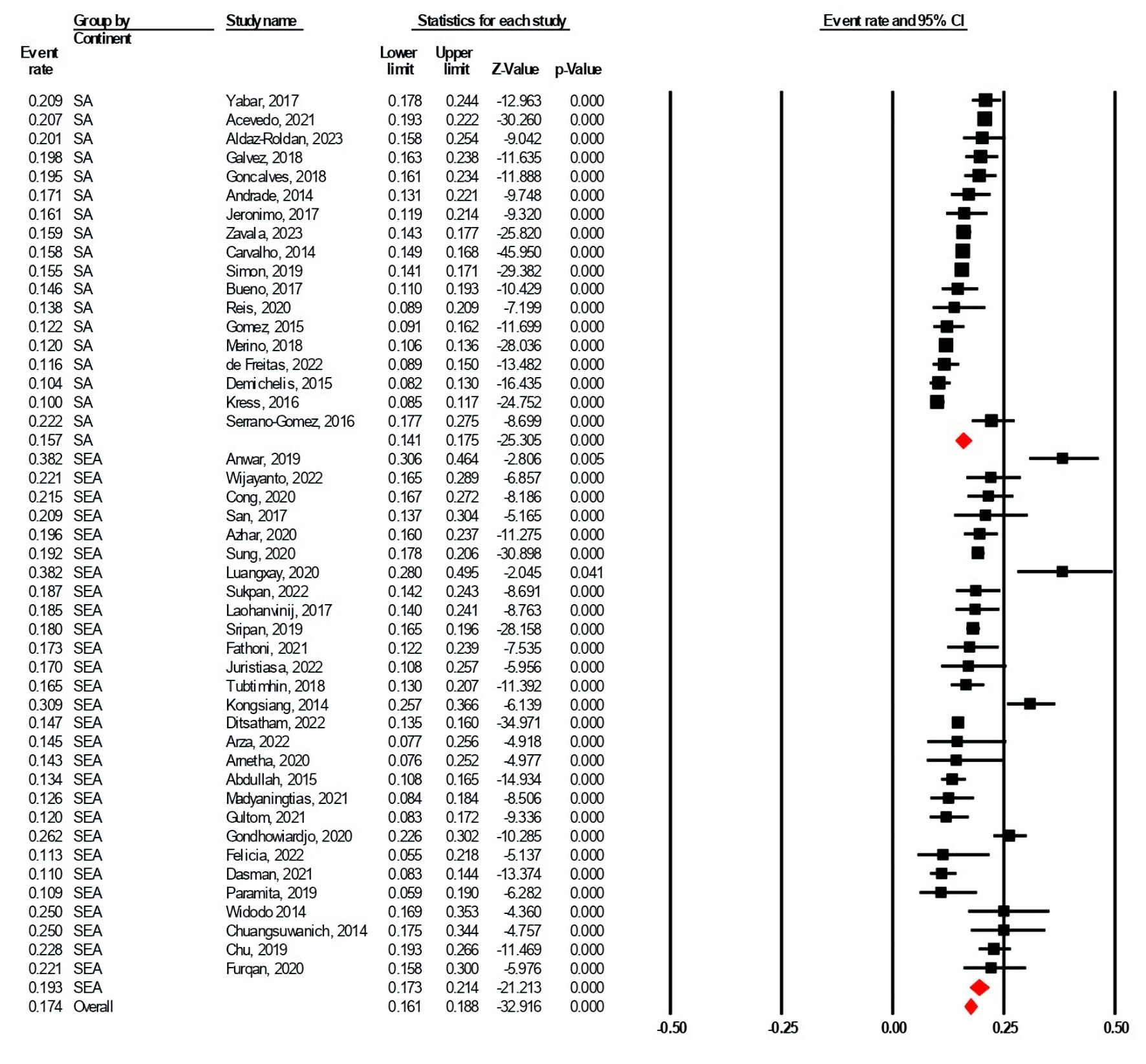 Click for large image | Figure 2. The TNBC subtype rate comparison in the SEA and the SA. The black squares and red diamond represent individual studies (countries) and continents, respectively, whereas the X-axis represented the TNBC rate per total BC population (0.00 to 1.00 for 0% to 100%). SEA: South-East Asia; SA: South America; CI: confidence interval; TNBC: triple-negative breast cancer; BC: breast cancer. |
SEA analysis
The SEA-specific analysis is generally portrayed on the Figure 3a, revealing that Laos might possess the highest rate of TNBC (38.2% (28.0-49.5%)), yet it was reported by only one single study by Luangxay et al [30], in 2019, with only 76 individuals. It was followed by Vietnam and Myanmar with the reported TNBC rate of 22.4% (19.5-25.5%; 738 individuals), and 20.9% (13.7-30.4%; 91 individuals), respectively. Thailand placed next with 19.6% (16.2-23.4%), followed by Indonesia with 17.8% (14.4-21.9%), both supported by high number of patients (2,788 and 6,685 individuals, respectively). Malaysia holds the lowest number of TNBC rate, with 16.3% (11.4-22.8%) estimation followed by a considerable sample size (3,539 individuals). However, the authors failed to identify eligible reports from five countries, i.e., Brunei, Cambodia, East Timor, Philippines, and Singapore, which might be originated from limited investigations from the countries, or it does not meet our applied criterion.
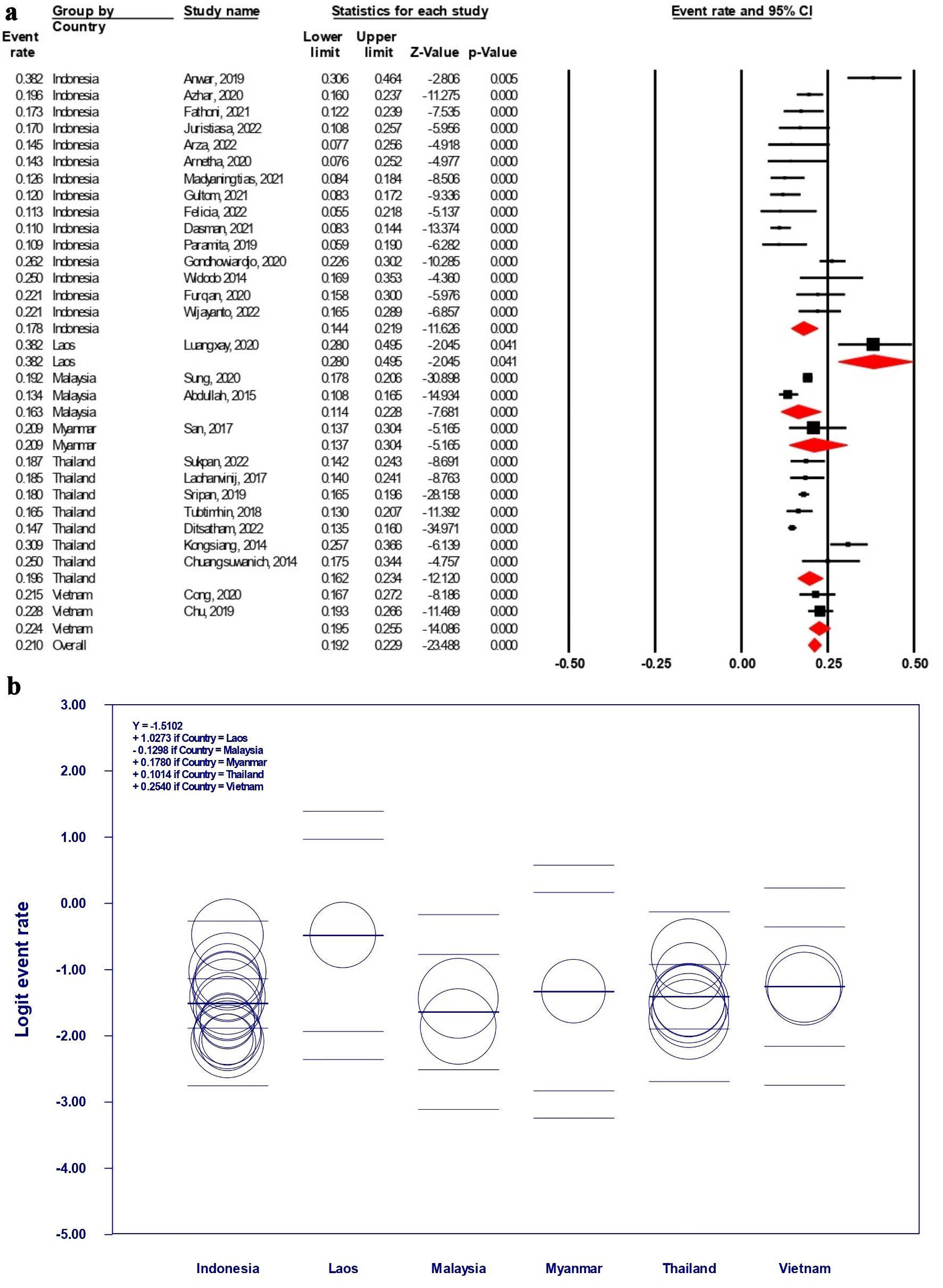 Click for large image | Figure 3. (a) The TNBC subtype rate estimation in SEA countries. (b) Scatterplot representation of logit event rate regression model among SEA countries. (a) The black squares and red diamonds represent individual studies (countries) and continents, respectively, whereas the X-axis represented the TNBC rate per total BC population (0.00 to 1.00 for 0% to 100%). (b) Each circle represents an individual study, whereas the thick line on the middle of each country section represents the estimated logit event rate. SEA: South-East Asia; CI: confidence interval; TNBC: triple-negative breast cancer; BC: breast cancer. |
The meta-regression of logit event rate from the SEA-based data is also presented in Figure 3b. As no overlapping of the event rates was present as proven by its lower- and upper-limit estimation, the difference of the countries’ TNBC rate was initially considered insignificant (predicted P > 0.05 for inter-country difference). However, we observed that there are several significant differences between countries such as Indonesia and Laos (P = 0.015), Malaysia and Laos (P = 0.015), and Thailand and Laos (P = 0.032). Other comparisons were not described further in this section as we estimated the P value to be > 0.05. It should be cautiously noted that Laos only possesses 76 BC samples in this study, remarkably lower than the other SEA countries, hence it might introduce some degree of biases. We suggest interpreting the outcomes carefully, considering that the observed significance within Laos may originated from its lack of samples; whilst sub-analysis to other SEA countries with larger sample size remains statistically acceptable.
SA analysis
Our analysis on the SA-specific analysis (Fig. 4a) demonstrated that Ecuador holds the highest TNBC rate of 20.1% (17.8-24.4%), though it only had one single study comprising 268 individuals. It was followed by Peru with 17.8% (15.1-20.9%) from 3,089 BC patients, Colombia with 16.7% (9.0-28.9%) from 607 BC patients, and Chile with 15.9% (9.1-26.3%) from 4,967 BC patients. Brazil with the highest patients count (9,473 individuals) reported TNBC rate of 15.8% (14.5-17.2%), which was estimated from seven different studies in several cities or regions in the country. Interestingly, there is a significant jump in Argentina’s TNBC rate, as it was estimated to be the lowest in SA with only 10.1% (8.9-11.5%).
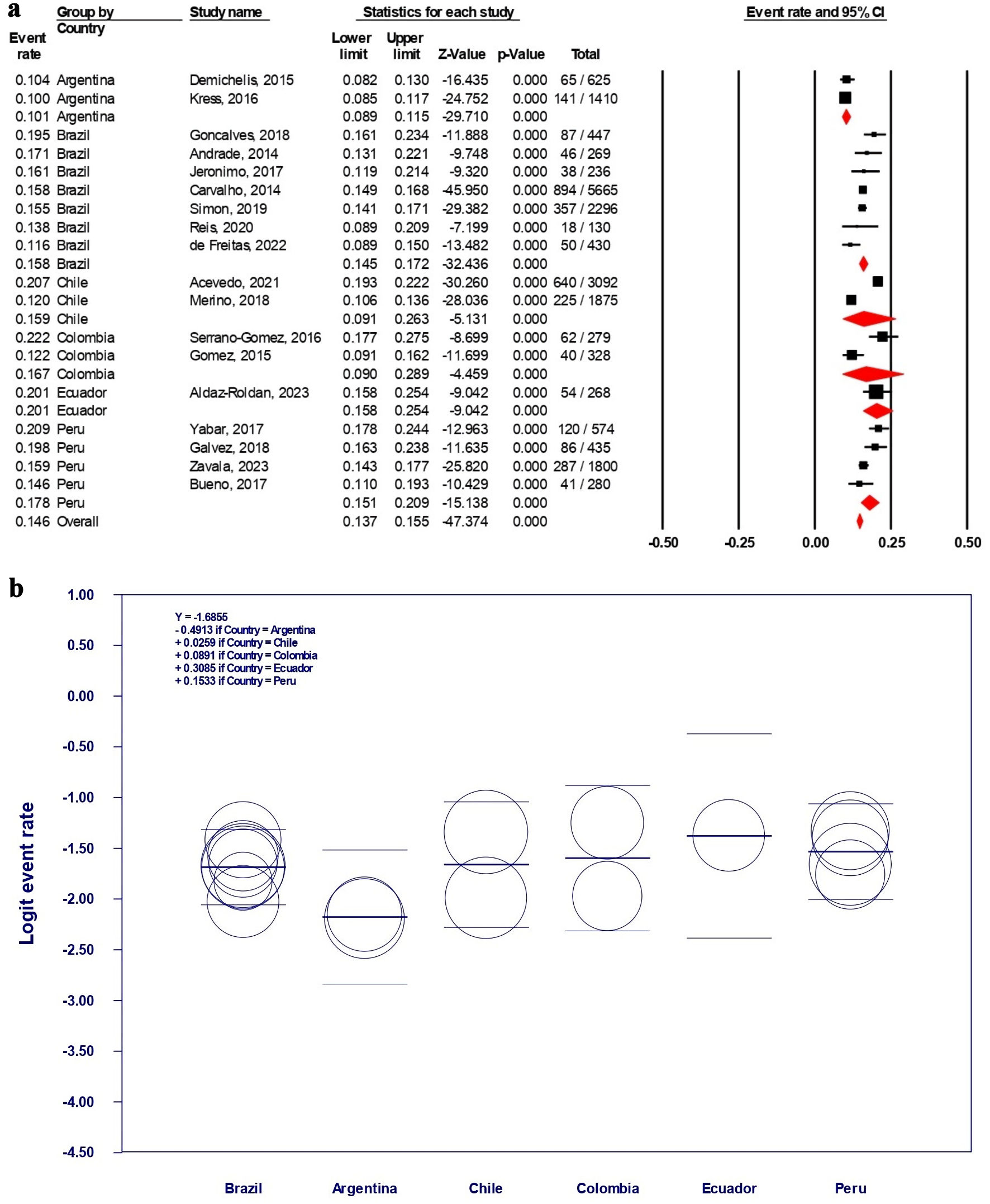 Click for large image | Figure 4. (a) The TNBC subtype rate estimation in SA countries. (b) Scatterplot representation of logit event rate regression model among SA countries. (a) The black squares and red diamonds represent individual studies (countries) and continents, respectively, whereas the X-axis represents the TNBC rate per total BC population (0.00 to 1.00 for 0% to 100%). (b) Each circle represents an individual study, whereas the thick line on the middle of each country section represents the estimated logit event rate. SA: South America; TNBC: triple-negative breast cancer; CI: confidence interval; BC: breast cancer. |
The forest plot of the SA-specific analysis disclosed that initially, the overlapping of the event rates was observed only in Argentina’s TNBC rate lower- and upper-limit when it was compared to other SA countries (predicted P < 0.05 for analysis related to Argentina). This prediction was proven by analyzing the meta-regression model’s construction (Fig. 4b), which demonstrated several significant findings related to Argentina, i.e., to Brazil (P = 0.022), to Chile (P = 0.043), to Colombia (P = 0.035), to Ecuador (P = 0.019), and to Peru (P = 0.005). Only Argentina possessed significant differences compared to other SA countries, as no other inter-SA country comparison demonstrated the P value of < 0.05, representing its observably lower TNBC rate among others.
| Discussion | ▴Top |
The basic principle of this review is to provide a generalized and wide-scoping meta-analysis on the TNBC distribution in both SEA and SA continents, hence we may capture the true epidemiological scale of the subtypes, and why it is necessary to extend the trials to the regions. We focused on the TNBC rate per total BC diagnosis, hence it is possible to estimate “how common a center may encounter those aggressive types in practice” statistically. Our work may also be able to be transcribed toward other issues in oncologic science, either by simply describing the histopathology-epidemiology characteristics of breast malignancy in the regions, or by any chance influencing the maneuver of healthcare provider in each country plus large pharmaceutical companies. To exemplify the benefit of this study in issuing the imbalance of trial-patient rate, the US National Library of Medicine only recorded a single-active trial of TNBC in Indonesia; though Indonesia is placing the fourth in total population (2023 report by the Worldometer, 49.7% is female; approximately ± 135 million individuals) [6, 61]. We also estimated that 17.8% of the BC diagnosis in Indonesia is triple-negative on IHC [12-26]. Supported by the GLOBOCAN 2020 country-specific report, at least there was 65,858 total BC cases (in which 11,000 - 12,000 of the patients are estimated to be TNBC on testing) [1]. We attempted to depict our findings in schematics (Figs. 5, 6), representing SEA and SA, respectively, therefore it will be much easier to identify the TNBC rate based on each report (denoted by the first author’s last name, sample size by each circle, and investigations’ period and location by city as pointed on the maps).
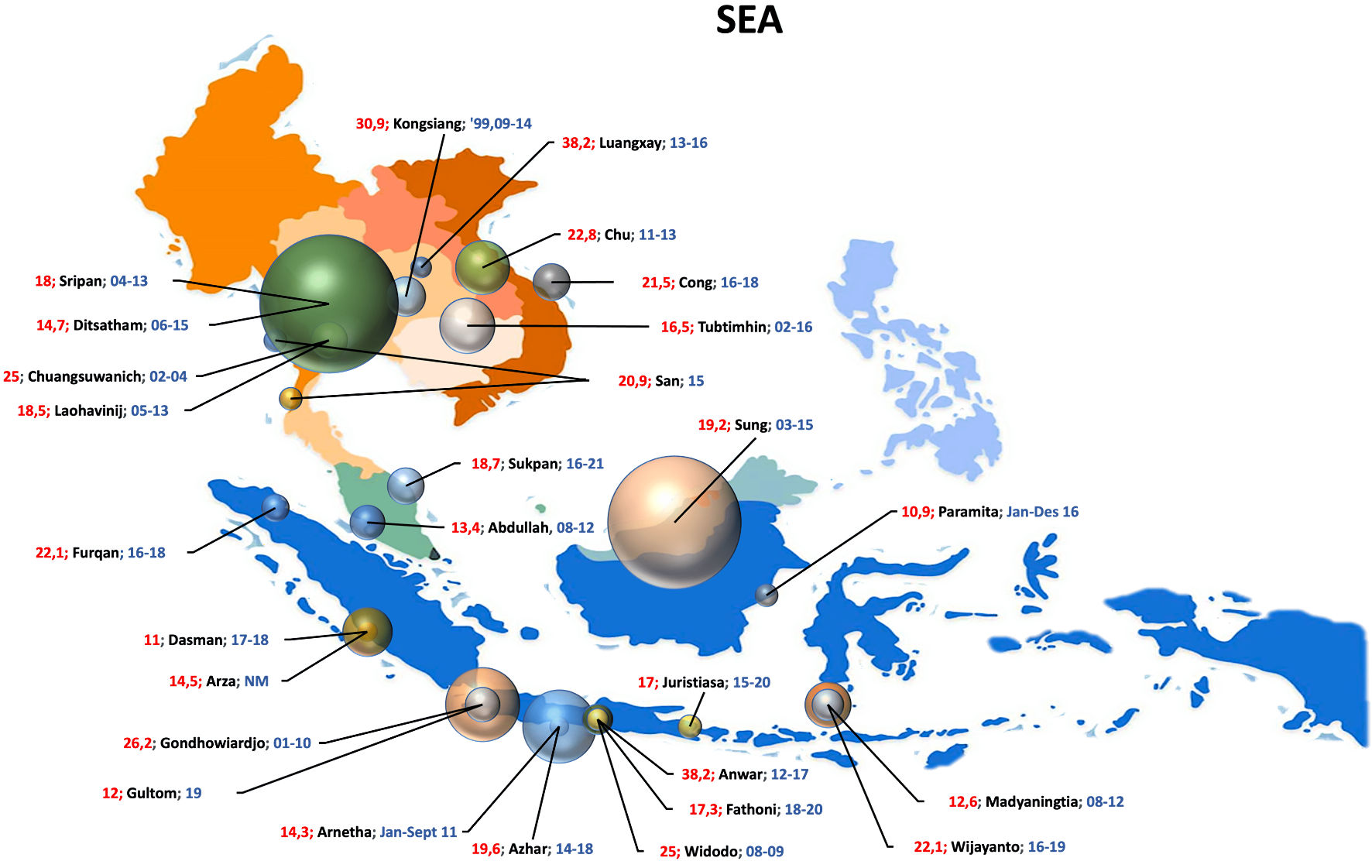 Click for large image | Figure 5. Schematic representation of the TNBC percentage and population size in SEA region. The numbers in red and blue represent TNBC percentage and year of observation (for example, “19.6; Azhar; 14-18” may stands for a study by Azhar et al, with TNBC rate of 19.6% and observation year of 2014 - 2018); respectively. TNBC: triple-negative breast cancer; SEA: South-East Asia. |
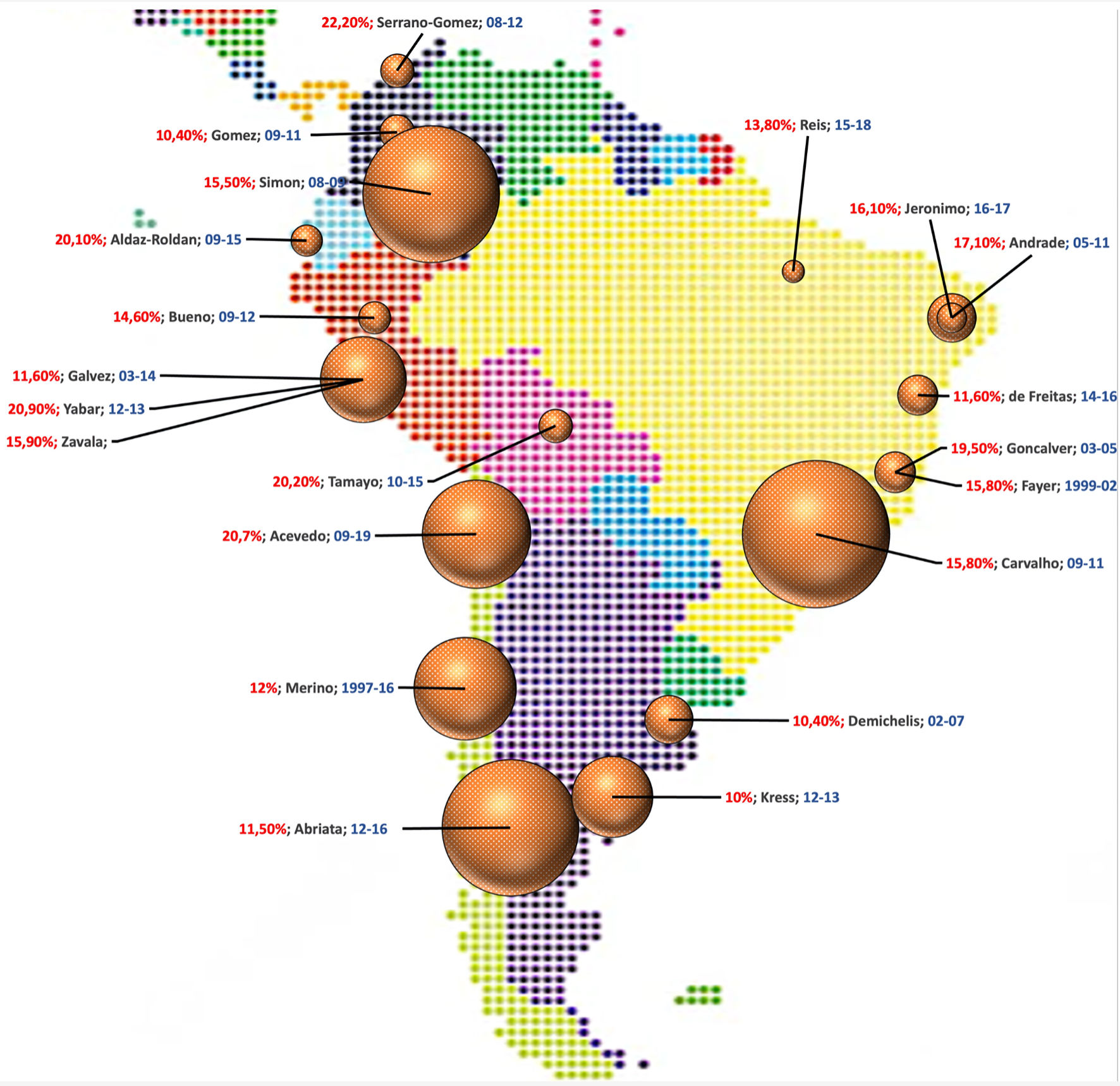 Click for large image | Figure 6. Schematic representation of the TNBC percentage and population size in SA region. The numbers in red and blue represent TNBC percentage and year of observation (for example “16.1; Jeronimo; 16-17” may stands for a study by Jeronimo et al, with TNBC rate of 16.1% and observation year of 2016 - 2017), respectively. TNBC: triple-negative breast cancer; SA: South America. |
Our estimation in both continents basically remains in line with the general prediction on the TNBC rate, which falls within the range of 15-25% [3, 4]. However, compared to other high-frequent trials countries (e.g., USA with 12% rate) which may be varied among reports (Dietze et al also estimated that the values among European-American vs. African American were 16% and 30%, respectively), the TNBC rate in our analyzed continents is proportional to global estimate [62, 63]. Though the European-based estimation of TNBC rate is not available at the moment, the close linkage between Europe and USA in term of the majority population should be considered, since ± 60% of the population was identified as European ancestry (or Caucasian) [64]. However, population with African ancestry was considered to possess higher risk of developing TNBC, which eventually worsened its prognostic. It is also believed that the TNBC rate may even reach > 40.0% limit in parts of Africa, underlining its common ground in genetics and raising the awareness to conduct region-based investigations [65, 66]. The genetic susceptibility of the Black population also shares the same idea to determine whether the collective ethnic groups in SEA (which mainly consisted of Austro-Asiatic, Austronesian, Negrito, etc.), or SA (which is often considered to be a mixture of individuals from multi-ethnic roots) may offer similar phenomenon occurred among the African descents [67, 68].
Nevertheless, considering the current global community demonstrating high societal diversity, the ethnic status was often overlooked as group-based studies, which eventually produce less satisfactions due to plurality of the subjects’ ethnic roots. In other hand, populations in either SEA or SA are plural by nature, hence attempt to homogenizing the individuals should be exempted. Consequently, confirming the genetic vulnerability should never be performed solely through ethnic identification, as it is recommended to conduct a genome-wide association analysis on specific loci (although we never fully contradict the role of ethnic roots on its association) [69, 70]. The modern medicine might need to reconsider the functionality of race and ethnicity in representing genetics due to their basic nature as a social construct, which is conceptually different with gene as a basic unit of heredity. The gene transfers the trait of an individual, often recognized as ethic characteristics in a population-scale judgment (or phenotype), whilst gene truthfully manifested the genotype aspect as well [71]. Interpreting our studies based on the modern point-of-view of population under a flag should be preferred, though the limitation in utilizing the data from this review is basically absent. It might as well represent the non-modifiable risk factors from each country, as it is also widely accepted that the interacting environmental factors play immense role in cancers’ pathogenesis [72, 73].
However, the lateral comparison of our findings should be made to the other continent or its equal (in term of population size) level review. Upon in-depth analysis on the findings, it was revealed that Laos and Argentina stand out to be different among their counterparts, with the Laos possessed the highest TNBC rate in SEA, but Argentina was the lowest of all in SA [30, 43, 74]. However, it is important to emphasize that we are only able to identify a single study from Laos that consisted of 76 patients. Vietnam follows in the second place with 20.9% rate from 738 individuals, though no significant difference was observed compared to the other SEA countries [41, 42]. Argentina in other hand, was estimated to have a 10.1% rate from 2,035 individuals across two studies, yet this study also demonstrated significant TNBC rate compared to the other SA countries (does not closely followed by Brazil on the second place with 15.8% rate) [43, 44, 46-51, 74]. This observation produces valuable insights on how both Argentina and Laos (or Vietnam) emerged from the pool. Is it originated from the genetic ancestry of its major population (which may be unable to be proven in our review), or perhaps it highly favors the nature of environmental risk factors in influencing carcinogenesis? One possible point of understanding is Argentina might possess lower rate of native American and consequently comprised of higher European descent population, unlike the other SA countries, i.e., Ecuador, Peru, etc. [75]. In other hand, capturing the relation between ancestry and cancer risk in SA region remains a significant challenge, considering the genetic admixture of the population is proven to be diverse as a collective origin pool of European, African, and Native American roots [76]. Nevertheless, we are able to recognize remarkable points in both Argentina and Laos, whilst possibly introducing the necessity to intensify the TNBC-trial numbers in those countries.
We also aspire to shape the future of the oncologic research in the reviewed regions, as most of the times, the stakeholders spent larger attentiveness on the Western population as represented by the trials’ number conducted. Regulating the TNBC markets should also consider both continents to be represented, as well in the populations, as many novel agents or even classes are being evaluated to date, yet very few trials have reached these regions. And yet, the most reasonable approaches to response our findings are to encourage higher TNBC trial recruitments, specifically among the evaluated region with higher disease rate per total BC population. Boosting the participant recruitments on TNBC trials from those nations will be the first step to keep up with significantly higher data availability from the Western population. Apart from the more favorable situation among the Western world for conducting trials, we believe that our study should be sufficient as a groundwork review to plan a priority list of upcoming trials based on the estimated TNBC rate.
The combination of pembrolizumab plus anthracycline, platinum agents, and taxane as a neoadjuvant regiment is the current standard approach for TNBC, followed by the individualized response or the BRCA gene testing results. It will eventually lead to much complex choices involving olaparib (and its derivatives from poly adenosine diphosphate (ADP) ribose polymerase (PARP) inhibitors), or other immune checkpoint inhibitors (ICIs), e.g., nivolumab, atezolizumab, etc., serving the role of personalized treatment in modern oncology [77, 78]. Despite the advancement of those classes as a whole, further exploration revealed that their administration was only effective on specific individuals, as determined by the BRCA status and the programmed death-ligand 1 (PD-L1) expressivity. Therefore, the landscape was even more limited among populations with metastatic lesion, without mutations of germline BRCA, or negatively expressing PD-L1 (< 1%), as sacituzumab govitecan being the only key agent to improve outcomes according to the phase III ASCENT trials [79, 80]. Interestingly, other trials involving trastuzumab deruxtecan (a HER-2 blocker), e.g., DAISY and Destiny-Breast06 (Destiny-06), also included HER2 non-expressing participants. Whilst it may indirectly extend the research on TNBC groups, the most recent reports continue to include the hormone receptor-positive BC as the evaluated subsets [81, 82]. The upcoming exploration within the Enhertu study involving trastuzumab has also revealed a likelihood of including TNBC patients for its trial, possibly expanding the pathology treatment choice in the future [83].
It is recorded that currently, there are 131 active trials of pembrolizumab on TNBC. All records involve both USA and Europe, but only 5.3% and 7.6% of the trials were conducted in SEA and SA, respectively [84]. Regardless the fact that pembrolizumab is one of the most prominent ICI agents in TNBC guidelines, less than 10% of the trials conducted involved representatives from at least 600 million women in the SEA and the SA [6]. Out of the total recorded trials, the trial numbers conducted on the PD-L1-related evaluation were slightly higher, with 11.2% and 13.4% from SEA and SA, respectively [85]. Similar phenomena were also observed in 333 identified trials involving anthracycline, taxane, and platinum agents, with 5.7% and 8.4% of the reports incorporating the population from the evaluated continents, regardless the fact that those agents had been around for longer than most of the ICI agents [86]. On total estimation of the trials utilizing the ICI agents, we were only able to identify 145 active studies from the US National Library of Medicine, which mentioned that at least 5.5% and 7.6% of the trials were regulated in both SEA and SA, with USA dominating the field with 73.8% of total reports [87]. For the record, the estimation was made based on the data provided on its website, aiming to at least represent the lower trial numbers conducted in both continents. However, we agreed that either the genetic susceptibility or the populations’ lifestyles are greatly distinct from the Western countries.
The growing concern in obtaining better and attainable cancer treatment for better oncologic care is the main intention in conducting this review. We have proven that the TNBC rate in countries from the reviewed continents is similar to or even relatively higher than the one of USA or Europe (though the authors have yet to provide its comparison with other Asian countries or the data from the Africa continent). The potential end-users of the standard TNBC medication and the novel ICIs agents, e.g., cancer-referral hospital, oncology clinic or centers, and even retail clinics, are available as well on those countries. Moreover, ongoing approach on the personalized medicine as the future of disease-eliminating effort should prioritize the genetic or at least regionalized investigations as the mutual understanding between pharma companies and each countries’ governmental stakeholders. Our review will accommodate the national or global attempt to capture the estimated TNBC rate and explore its implication for the populations’ requirement. Utilizing our review to seize the status quo of the TNBC rate may positively influence the decision making or improve the accessibility of cancer care in those countries, encouraging each party that the urgencies to conduct further trials are prevailed.
This meta-analysis holds a number of limitations that should be considered before interpreting the outcomes. First of all, we establish a “one period, one center or city” policy to decrease the risk of reporting biases; hence often studies of smaller population that overlapped with the policy were excluded from the final analysis. We also recognized the abstraction of our review since a plethora of discussions may occur from every finding in this study, which might as well can be considered as this review’s main strength. Next, the authors transcribed the significance of each country’s differences based on the regression of logit event rate (presented by two-sided P value in 95% CI). Therefore, interpretation of the outcomes was structured from the estimated P value plus qualitative assessment on the scatterplot models; and it will be very much appreciated to receive feedback from our modus operandi in delivering the objectives. For that reason, we are highly open for discussion to improve the level of this meta-analysis by approaching the corresponding author (DH), considering the analysis might be limited by the authors’ statistical performance in interpreting the outcomes, and we aimed to propose another review with similar model on the specific estrogen or progesterone receptors’ expression rate.
| Conclusions | ▴Top |
This study highlights the epidemiological estimation of TNBC’s prevalence on both SEA and SA regions, with specific consideration that SEA has higher TNBC rate compared to the SA populations in this review. We estimated percentage estimations, with some countries, i.e., Argentina or even Laos possess significant difference compared to the respective countries’ neighbors. Nevertheless, better understanding on the current TNBC epidemiological status in the majority-improving countries from our review might theoretically improve cancer care, either by encouraging the government as the primary healthcare stakeholders or the trials-leading pharma companies. As we are able to slightly portray the imbalances of trials-patients ratio in both SEA and SA compared to the Western countries, this study might as well serve as a large-scale guide of epidemiological investigation in those continents. Consequently, our finding may also encourage TNBC trials’ recruitment on the reviewed regions, considering the TNBC epidemiological status had been partially captured in this study.
| Supplementary Material | ▴Top |
Suppl 1. Risk of bias assessment of the studies in Newcastle-Ottawa scale.
Acknowledgments
The authors express gratitude to the Korean Society of Medical Oncology (KSMO) for accepting the abstract version of this study in their 2023 annual congress, allowing the authors to improve the reachability of our work to the global oncologic societies.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Author Contributions
The CRediT taxonomy captured each author’s contribution in the preparation of this work: Conceptualization: DH, NNF. Data curation: DH, NNF, RHNS. Formal analysis: DH, NNF, RHNS. Funding acquisition: Not applicable. Investigation: DH, NNF, RHBS. Methodology: DH, NNF, RHNS, NND. Project administration: NNF, RHNS. Resources: DH, NNF. Software: DH, NNF, RHNS, NND. Supervision: DH. Validation: DH. Visualization: NNF, RHNS, NND. Writing - original draft: DH, NNF. Writing - review and editing: DH, NNF, RHNS.
Data Availability
All data and material are available on request by contacting the corresponding or first author. The inquiries will be considered after thorough evaluation.
| References | ▴Top |
- Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209-249.
doi pubmed - Zagami P, Carey LA. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer. 2022;8(1):95.
doi pubmed pmc - Yin L, Duan JJ, Bian XW, Yu SC. Triple-negative breast cancer molecular subtyping and treatment progress. Breast Cancer Res. 2020;22(1):61.
doi pubmed pmc - Almansour NM. Triple-negative breast cancer: a brief review about epidemiology, risk factors, signaling pathways, treatment and role of artificial intelligence. Front Mol Biosci. 2022;9:836417.
doi pubmed pmc - US National Library of Medicine. Trials of triple negative breast cancer [Internet]. Search Results. 2023 [cited Dec 1, 2023]. Available from: https://classic.clinicaltrials.gov/ct2/results?recrs=abdef&cond=Triple+Negative+Breast+Cancer.
- The World Bank. Female [Internet]. Population. 2022 [cited Dec 1, 2023]. Available from: https://data.worldbank.org/indicator/SP.POP.TOTL.FE.IN?end=2022&start=1960&view=chart.
- Kim CS, Lee S. Different Paths of Deindustrialization: Latin American and Southeast Asian Countries from a Comparative Perspective. J Int Area Stud [Internet]. 2014;21(2):65-81.
- Tsunekawa K. Emerging States in Latin America: how and why they differ from their Asian counterparts. In: Tsunekawa K, Todo Y, editors. Emerging states at crossroads: emerging-economy state and international policy studies. Tokyo: Springer Open; 2019. p. 71-96.
- Skoglund P, Mallick S, Bortolini MC, Chennagiri N, Hunemeier T, Petzl-Erler ML, Salzano FM, et al. Genetic evidence for two founding populations of the Americas. Nature. 2015;525(7567):104-108.
doi pubmed pmc - Firsty NF, Hermansyah D, Siagian RH, Dwinda ND. Intercontinental Comparison of Immunohistochemical Subtypes among Individuals with Breast Cancer in South-East Asia and South America: Scoping Systematic Review and Meta-Analysis of Observational Studies [Internet]. PROSPERO: International prospective register of systematic reviews. 2023 [cited Dec 19, 2023]. Report No.: CRD42023466295. Available from: https://www.crd.york.ac.uk/prospero/export_details_pdf.php.
- Ribeiro CM, Beserra BTS, Silva NG, Lima CL, Rocha PRS, Coelho MS, Neves FAR, et al. Exposure to endocrine-disrupting chemicals and anthropometric measures of obesity: a systematic review and meta-analysis. BMJ Open. 2020;10(6):e033509.
doi pubmed pmc - Agency for Healthcare Research and Quality. Guidelines for Ensuring the Quality of Information Disseminated to the Public [Internet]. Research. 2015 [cited Jan 25, 2024]. Available from: https://www.ahrq.gov/research/publications/ahrq-info-quality-gdlns.html.
- Brüggemann P, Rajguru K. Comprehensive Meta-Analysis (CMA) 3.0: a software review. J Mark Anal [Internet]. 2022;10(4):425-429.
- Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta Analysis Version 3.0 [Internet]. Meta-Analysis Manual V3. 2007 [cited Dec 19, 2023]. Available from: https://meta-analysis.com/download/Meta-Analysis Manual V3.pdf.
- Arnetha TS, Hernowo BS, Adha MJ, Rezano A. Relationship between molecular subtypes and overall survival of breast cancer in Bandung. Biomed Pharmacol J. 2020;13(3):1543-1548.
- Azhar Y, Agustina H, Abdurahman M, Achmad D. Breast cancer in West Java: where do we stand and go? Indones J Cancer. 2020;14(3):91.
- Juristiasa DPS, Adiputra PAT, Sukrama DM. Survival analysis of patients with luminal and non-luminal subtype breast cancer receiving vinorelbine therapy at Sanglah General Hospital, Denpasar, Bali. Indones J Cancer. 2022;16(2):100.
- Felicia D, Hellyanti T. Stromal tumor infiltrating lymphocytes (sTIL) as an independent predictor of pathologic response to neadjuvant chemotherapy in breast cancer in Indonesia: a hospital-based study. Asian Pac J Cancer Prev. 2022;23(8):2763-2769.
doi pubmed pmc - Gondhowiardjo S, Soediro R, Jayalie VF, Djoerban Z, Siregar NC, Poetiray EDC. Multicenter management of breast cancer in indonesia: ten years of experience. eJournal Kedokt Indones. 2020;8(2):121-130.
- Gultom FL, Nurprilinda M, Hutami RNC. Immunohistochemical profiles of breast cancer patients at MRCCC Siloam Semanggi Hospital in 2018. Int J Heal Sci Res. 2021;11(5):392-400.
- Madyaningtias E, Sampepajung D, Prihantono P, Faruk M. Epidemiological and clinicopathological characteristics of breast cancer in Eastern Indonesia. J Med Allied Sci. 2021;11(1):27.
- Wijayanto A, Pieter JSLA, Prihantono P, Syamsu SA, Thaufix NS, Abdi A. Survivability rates based on molecular subtype, stage and metastasis of 36 months cohort in breast cancer patients. Nusant Med Sci J. 2022;7(1):29-38.
- Furqan M, Pohan PU. Relationship of Histopathology Grading with Molecular Subtypes of Breast Cancer Patients in Haji Adam Malik General Hospital 2016-2018. Scr SCORE Sci Med J. 2020;2(1):28-37.
- Arza IA, Daan Khambri, Rony Rustam, Hera Novianti, Husna Yetti. Relationship between molecular subtype, degree of differentiation, and lymph node metastasis with survival of breast cancer patients at Dr. M. Djamil General Hospital, Padang, Indonesia. Biosci Med J Biomed Transl Res. 2022;6(16):2779-2783.
- Dasman H, Harahap WA, Khambri D. Clinicopathology profiles and outcome of breast cancer in Indonesian National Health Insurance Patients. International Journal of Oncology and Cancer Therapy. 2021;5:1-8.
- Paramita S, Raharjo EN, Niasari M, Azizah F, Hanifah NA. Luminal B is the most common intrinsic molecular subtypes of invasive ductal breast carcinoma patients in East Kalimantan, Indonesia. Asian Pac J Cancer Prev. 2019;20(8):2247-2252.
doi pubmed pmc - Anwar SL, Raharjo CA, Herviastuti R, Dwianingsih EK, Setyoheriyanto D, Avanti WS, Choridah L, et al. Pathological profiles and clinical management challenges of breast cancer emerging in young women in Indonesia: a hospital-based study. BMC Womens Health. 2019;19(1):28.
doi pubmed pmc - Fathoni MIA, Gunardi, Adi-Kusumo F, Hutajulu SH, Purwanto I. Characteristics of breast cancer patients at dr. Sardjito Hospital for early anticipation of neutropenia: Cross-sectional study. Ann Med Surg (Lond). 2022;73:103189.
doi pubmed pmc - Widodo I, Dwianingsih EK, Triningsih E, Utoro T, Soeripto. Clinicopathological features of indonesian breast cancers with different molecular subtypes. Asian Pac J Cancer Prev. 2014;15(15):6109-6113.
doi pubmed - Luangxay T, Virachith S, Hando K, Vilayvong S, Xaysomphet P, Arounlangsy P, Phongsavan K, et al. Subtypes of breast cancer in Lao P.D.R.: a study in a limited-resource setting. Asian Pac J Cancer Prev. 2019;20(2):589-594.
doi pubmed pmc - Sung H, Devi BCR, Tang TS, Rosenberg PS, Anderson WF, Yang XR. Divergent breast cancer incidence trends by hormone receptor status in the state of Sarawak, Malaysia. Int J Cancer. 2020;147(3):829-837.
doi pubmed pmc - Abdullah MM, Mohamed AK, Foo YC, Lee CM, Chua CT, Wu CH, Hoo L, et al. Breast cancer survival at a leading Cancer Centre in Malaysia. Asian Pac J Cancer Prev. 2015;16(18):8513-8517.
doi pubmed - San TH, Fujisawa M, Fushimi S, Soe L, Min NW, Yoshimura T, Ohara T, et al. Molecular subtypes of breast cancers from Myanmar women: a study of 91 cases at two pathology centers. Asian Pac J Cancer Prev. 2017;18(6):1617-1621.
doi pubmed pmc - Chuangsuwanich T, Pongpruttipan T, P OC, Komoltri C, Watcharahirun S, Sa-Nguanraksa D. Clinicopathologic features of breast carcinomas classified by biomarkers and correlation with microvessel density and VEGF expression: a study from Thailand. Asian Pac J Cancer Prev. 2014;15(3):1187-1192.
doi pubmed - van Ommen-Nijhof A, Steenbruggen TG, Capel L, Vergouwen M, Vrancken Peeters MT, Wiersma TG, Sonke GS. Survival and prognostic factors in oligometastatic breast cancer. Breast. 2023;67:14-20.
doi pubmed pmc - Ditsatham C, Sripan P, Chaiwun B, Klunklin P, Tharavichitkul E, Chakrabandhu S, Muangwong P, et al. Breast cancer subtypes in Northern Thailand and Barriers to satisfactory survival outcomes. BMC Cancer. 2022;22(1):1147.
doi pubmed pmc - Sripan P, Sriplung H, Pongnikorn D, Bilheem S, Virani S, Waisri N, et al. Clinical subtypes of breast cancer in Thai women: A population-based study of Chiang Mai province. Asian Biomed. 2019;13(1):11-17.
- Kongsiang A, Tangvoraphonkchai V, Jirapornkul C, Promthet S, Kamsa-Ard S, Suwanrungruang K. Survival time and molecular subtypes of breast cancer after radiotherapy in Thailand. Asian Pac J Cancer Prev. 2014;15(23):10505-10508.
doi pubmed - Sukpan P, Kanokwiroon K, Sriplung H, Paisarn P, Sangkhathat S. Survival outcomes in breast cancer patients and associated factors in a border province of Thailand: a hospital-based review. J Heal Sci Med Res. 2023;41(3):1-9.
- Tubtimhin S, Promthet S, Suwanrungruang K, Supaattagorn P. Molecular subtypes and prognostic factors among premenopausal and postmenopausal Thai women with invasive breast cancer: 15 years follow-up data. Asian Pac J Cancer Prev. 2018;19(11):3167-3174.
doi pubmed pmc - Nguyen VC, Nguyen TQ, Vu TNH, Phung TH, Nguyen TPH, Nguyen ND, Le DR. Application of St Gallen categories in predicting survival for patients with breast cancer in Vietnam. Cancer Control. 2019;26(1):1073274819862794.
doi pubmed pmc - Dang Cong T, Nguyen Thanh T, Nguyen Phan QA, Hoang Thi AP, Nguyen Tran BS, Nguyen Vu QH. Correlation between HER2 expression and clinicopathological features of breast cancer: a cross- sectional study in Vietnam. Asian Pac J Cancer Prev. 2020;21(4):1135-1142.
doi pubmed pmc - Pablo Meiss Kress R. Breast cancer in Argentina: analysis from a collaborative group for the study of female breast cancer. J Cancer Epidemiol Treat. 2016;5-16.
- Goncalves H, Jr., Guerra MR, Duarte Cintra JR, Fayer VA, Brum IV, Bustamante Teixeira MT. Survival study of triple-negative and non-triple-negative breast cancer in a Brazilian cohort. Clin Med Insights Oncol. 2018;12:1179554918790563.
doi pubmed pmc - Fayer VA, Guerra MR, Cintra JR, Bustamante-Teixeira MT. Ten-year survival and prognostic factors for breast cancer in the southeast region of Brazil. Rev Bras Epidemiol. 2016;19(4):766-778.
doi pubmed - Reis A, Teixeira CMS, Medeiros ARL, Chaves KZC, Albuquerque CR, Melo MR. Sociodemographic and clinical-pathological study of molecular subtitles of breast carcinoma in a reference unit of Maranhao. Rev Bras Ginecol Obstet. 2020;42(12):820-828.
doi pubmed pmc - de Freitas RM, Guerra MR, Fayer VA, Campos AAL, Cintra JRD, Warren J, Ervilha RR, et al. Histological and Immunohistochemical Characteristics for Hereditary Breast Cancer Risk in a Cohort of Brazilian Women. Rev Bras Ginecol Obstet. 2022;44(8):761-770.
doi pubmed pmc - de Macedo Andrade AC, Ferreira Junior CA, Dantas Guimaraes B, Pessoa Barros AW, Sarmento de Almeida G, Weller M. Molecular breast cancer subtypes and therapies in a public hospital of northeastern Brazil. BMC Womens Health. 2014;14:110.
doi pubmed pmc - Jeronimo AFA, Weller M. Differential association of the lifestyle-related risk factors smoking and obesity with triple negative breast cancer in a Brazilian population. Asian Pac J Cancer Prev. 2017;18(6):1585-1593.
doi pubmed pmc - Simon SD, Bines J, Werutsky G, Nunes JS, Pacheco FC, Segalla JG, Gomes AJS, et al. Characteristics and prognosis of stage I-III breast cancer subtypes in Brazil: The AMAZONA retrospective cohort study. Breast. 2019;44:113-119.
doi pubmed - Carvalho FM, Bacchi LM, Pincerato KM, Van de Rijn M, Bacchi CE. Geographic differences in the distribution of molecular subtypes of breast cancer in Brazil. BMC Womens Health. 2014;14:102.
doi pubmed pmc - Acevedo F, Petric M, Walbaum B, Robin J, Legorburu L, Murature G, Guerra C, et al. Better overall survival in patients who achieve pathological complete response after neoadjuvant chemotherapy for breast cancer in a Chilean public hospital. Ecancermedicalscience. 2021;15:1185.
doi pubmed pmc - Merino T, Ip T, Dominguez F, Acevedo F, Medina L, Villaroel A, Camus M, et al. Risk factors for loco-regional recurrence in breast cancer patients: a retrospective study. Oncotarget. 2018;9(54):30355-30362.
doi pubmed pmc - Gomez R, Ossa CA, Montoya ME, Echeverri C, Angel G, Ascuntar J, Borrero M, et al. Impact of immunohistochemistry-based molecular subtype on chemosensitivity and survival in Hispanic breast cancer patients following neoadjuvant chemotherapy. Ecancermedicalscience. 2015;9:562.
doi pubmed pmc - Serrano-Gomez SJ, Sanabria-Salas MC, Hernandez-Suarez G, Garcia O, Silva C, Romero A, Mejia JC, et al. High prevalence of luminal B breast cancer intrinsic subtype in Colombian women. Carcinogenesis. 2016;37(7):669-676.
doi pubmed pmc - Aldaz-Roldan P, Pardo-Vasquez DF, Chamba-Morales GN, Aguirre-Reyes DF, Castillo-Calvas JM, Noblecilla-Arevalo G. Immunohistochemical subtype and its relationship with 5-year overall survival in breast cancer patients. Ecancermedicalscience. 2023;17:1509.
doi pubmed pmc - Bueno GAM. [Clinical and prognostic characteristics of the molecular subtypes of breast cancer determined by immunohistochemistry. Arequipa, Peru]. Rev Peru Med Exp Salud Publica. 2017;34(3):472-477.
doi pubmed - Galvez M, Castaneda CA, Sanchez J, Castillo M, Rebaza LP, Calderon G, Cruz M, et al. Clinicopathological predictors of long-term benefit in breast cancer treated with neoadjuvant chemotherapy. World J Clin Oncol. 2018;9(2):33-41.
doi pubmed pmc - Zavala VA, Casavilca-Zambrano S, Navarro-Vasquez J, Tamayo LI, Castaneda CA, Valencia G, Morante Z, et al. Breast cancer subtype and clinical characteristics in women from Peru. Front Oncol. 2023;13:938042.
doi pubmed pmc - Yabar A, Melendez R, Munoz S, Deneo H, Freire J, Dominguez V, Carrasco-Navarro RM, et al. Effect of Ki-67 assessment in the distribution of breast cancer subtypes: Evaluation in a cohort of Latin American patients. Mol Clin Oncol. 2017;6(4):503-509.
doi pubmed pmc - Worldometer. Countries in the world by population [Internet]. Population by Country. 2023 [cited Dec 3, 2023]. Available from: https://www.worldometers.info/world-population/population-by-country/.
- Dietze EC, Sistrunk C, Miranda-Carboni G, O'Regan R, Seewaldt VL. Triple-negative breast cancer in African-American women: disparities versus biology. Nat Rev Cancer. 2015;15(4):248-254.
doi pubmed pmc - Howard FM, Olopade OI. Epidemiology of triple-negative breast cancer: a review. Cancer J. 2021;27(1):8-16.
doi pubmed - Statistical Atlas. Race and Ethnicity in the United States [Internet]. Demographics. 2023 [cited Dec 3, 2023]. Available from: https://statisticalatlas.com/United-States/Race-and-Ethnicity#google_vignette.
- Brewster AM, Chavez-MacGregor M, Brown P. Epidemiology, biology, and treatment of triple-negative breast cancer in women of African ancestry. Lancet Oncol. 2014;15(13):e625-e634.
doi pubmed pmc - Siddharth S, Sharma D. Racial Disparity and Triple-Negative Breast Cancer in African-American Women: A Multifaceted Affair between Obesity, Biology, and Socioeconomic Determinants. Cancers (Basel). 2018;10(12):514.
doi pubmed pmc - Britannica. Latin America [Internet]. Sociology & Society. 2023 [cited Dec 3, 2023]. Available from: https://www.britannica.com/topic/race-human/Latin-America.
- Britannica. People of Southeast Asia [Internet]. Geographic Regions. 2023 [cited Dec 3, 2023]. Available from: https://www.britannica.com/place/Southeast-Asia/People.
- Borrell LN, Elhawary JR, Fuentes-Afflick E, Witonsky J, Bhakta N, Wu AHB, Bibbins-Domingo K, et al. Race and genetic ancestry in medicine - a time for reckoning with racism. N Engl J Med. 2021;384(5):474-480.
doi pubmed pmc - Huang T, Shu Y, Cai YD. Genetic differences among ethnic groups. BMC Genomics. 2015;16:1093.
doi pubmed pmc - National Academies of Sciences, Engineering and M. Population descriptors in human genetics research: genesis, evolution, and challenges. In: Using population descriptors in genetics and genomics research: a new framework for an evolving field [Internet]. Washington DC: The National Academies Press.; 2023. Available from: http://nap.nationalacademies.org/26902.
- Coppede F. Genes and the environment in cancer: focus on environmentally induced DNA methylation changes. Cancers (Basel). 2023;15(4):1-5.
doi pubmed pmc - Mbemi A, Khanna S, Njiki S, Yedjou CG, Tchounwou PB. Impact of gene-environment interactions on cancer development. Int J Environ Res Public Health. 2020;17(21):1-15.
doi pubmed pmc - Demichelis SO, Cermignani L, Zwenger A, Giacomi N, Barbera A, Segal-Eiras A, et al. Breast cancer risk and prognostic factors in two Argentine settings. J Cancerol. 2015;2:131-139.
- Parolin ML, Toscanini UF, Velazquez IF, Llull C, Berardi GL, Holley A, Tamburrini C, et al. Genetic admixture patterns in Argentinian Patagonia. PLoS One. 2019;14(6):e0214830.
doi pubmed pmc - Chacon-Duque JC, Adhikari K, Fuentes-Guajardo M, Mendoza-Revilla J, Acuna-Alonzo V, Barquera R, Quinto-Sanchez M, et al. Latin Americans show wide-spread Converso ancestry and imprint of local Native ancestry on physical appearance. Nat Commun. 2018;9(1):5388.
doi pubmed pmc - Nunes Filho P, Albuquerque C, Pilon Capella M, Debiasi M. Immune checkpoint inhibitors in breast cancer: a narrative review. Oncol Ther. 2023;11(2):171-183.
doi pubmed pmc - Lee J. Current Treatment Landscape for Early Triple-Negative Breast Cancer (TNBC). J Clin Med. 2023;12(4):1524.
doi pubmed pmc - Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, Brufsky A, et al. Sacituzumab Govitecan in metastatic triple-negative breast cancer. N Engl J Med. 2021;384(16):1529-1541.
doi pubmed - Bartsch R, Rinnerthaler G, Petru E, Egle D, Gnant M, Balic M, Sliwa T, et al. Updated Austrian treatment algorithm for metastatic triple-negative breast cancer. Wien Klin Wochenschr. 2023.
doi pubmed - ClinicalTrials.gov. Study of Trastuzumab Deruxtecan (T-DXd) vs investigator’s choice chemotherapy in HER2-low, hormone receptor positive, metastatic breast cancer (DB-06) [Internet]. [cited Mar 1, 2024]. Available from: https://clinicaltrials.gov/study/NCT04494425.
- Mosele F, Deluche E, Lusque A, Le Bescond L, Filleron T, Pradat Y, Ducoulombier A, et al. Trastuzumab deruxtecan in metastatic breast cancer with variable HER2 expression: the phase 2 DAISY trial. Nat Med. 2023;29(8):2110-2120.
doi pubmed pmc - Harmaceutical Technology. Trastuzumab deruxtecan by Daiichi Sankyo for Triple-Negative Breast Cancer (TNBC): Likelihood of Approval [Internet]. Data Insights. 2024 [cited Apr 1, 2024]. Available from: https://www.pharmaceutical-technology.com/data-insights/trastuzumab-deruxtecan-daiichi-sankyo-triple-negative-breast-cancer-tnbc-likelihood-of-approval/?cf-view.
- US National Library of Medicine. Trials of Triple Negative Breast Cancer and Pembrolizumab [Internet]. Search Results. 2023 [cited Dec 5, 2023]. Available from: https://classic.clinicaltrials.gov/ct2/results/map?term=PD-L1&recrs=abdef&cond=Triple+Negative+Breast+Cancer&map=.
- US National Library of Medicine. Trials of Triple Negative Breast Cancer and PD-L1 [Internet]. Search Results. 2023 [cited Dec 6, 2023]. Available from: https://classic.clinicaltrials.gov/ct2/results/map?term=pembrolizumab&recrs=abdef&cond=Triple+Negative+Breast+Cancer&map=.
- US National Library of Medicine. Trials of Triple Negative Breast Cancer and Anthracycline, Taxane, and Platinum Agents [Internet]. Search Results. 2023 [cited Dec 6, 2023]. Available from: https://classic.clinicaltrials.gov/ct2/results/map?cond=Triple+Negative+Breast+Cancer&term=taxane+OR+platinum+OR+anthracycline+OR+carboplatin+OR+cyclophosphamide+OR+paclitaxel+OR++docetaxel+OR+epirubicin&cntry=&state=&city=&dist=&Search=Search&recrs=a&rec.
- US National Library of Medicine. Trials of Triple Negative Breast Cancer and Immune Checkpoint Inhibitors [Internet]. Search Results. 2023 [cited Dec 6, 2023]. Available from: https://classic.clinicaltrials.gov/ct2/results/map?term=pembrolizumab+OR+secukinumab+OR+tocilizumab+OR+rituximab+OR+infliximab+OR+alemtuzumab+OR+natalizumab+OR+%22sacituzumab+govitecan%22&recrs=abdef&cond=Triple+Negative+Breast+Cancer&map=.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


