| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 15, Number 3, June 2024, pages 511-520
Complete Pathologic Response to Gemcitabine and Oxaliplatin Chemotherapy After Prior Therapies in a Patient With Hepatocellular Carcinoma and Peritoneal Metastases Undergoing Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy
Amry Majeeda, Sneha Alaparthib, Dina Halegoua-DeMarzioc, Jaime Eberle-Singhd, Wei Jiangd, Pramila Rani Annee, Ashesh P. Shahb, Wilbur B. Bowneb, Daniel Linf, g
aDepartment of Internal Medicine, Thomas Jefferson University Hospital, Philadelphia, PA, USA
bDepartment of General Surgery, Thomas Jefferson University Hospital, Philadelphia, PA, USA
cDivision of Gastroenterology and Hepatology, Department of Internal Medicine, Thomas Jefferson University Hospital, Philadelphia, PA, USA
dDepartment of Pathology and Genomic Medicine, Thomas Jefferson University Hospital, Philadelphia, PA, USA
eDepartment of Radiation Oncology, Thomas Jefferson University Hospital, Philadelphia, PA, USA
fDepartment of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University Hospital, Philadelphia, PA, USA
gCorresponding Author: Daniel Lin, Department of Medical Oncology, Sidney Kimmel Cancer Center, Thomas Jefferson University Hospital, Philadelphia, PA 19107, USA
Manuscript submitted February 10, 2024, accepted May 1, 2024, published online May 7, 2024
Short title: GEMOX Complete Response Hepatocellular Cancer
doi: https://doi.org/10.14740/wjon1840
| Abstract | ▴Top |
Hepatocellular carcinoma (HCC) is often diagnosed at a late stage and frequently recurs despite curative intervention, leading to poor survival outcomes. Frontline systemic therapies include combination immunotherapy regimens and tyrosine kinase inhibitors. We report a case of a 38-year-old woman with chronic hepatitis B and C coinfection-associated non-cirrhotic HCC, which recurred in the peritoneum after initial resection of her primary tumor. Disease progression occurred on both atezolizumab/bevacizumab and lenvatinib, and she was subsequently treated with gemcitabine and oxaliplatin (GEMOX) chemotherapy and exhibited a profound clinical response on imaging with normalization of alpha fetoprotein (AFP) after several months. Following extensive multidisciplinary discussion, she underwent cytoreductive surgery (CRS) with hyperthermic intraperitoneal chemotherapy (HIPEC) that removed all visible macroscopic tumor. Her pathology demonstrated a complete pathologic response. She received two additional months of postoperative chemotherapy, and then proceeded with close monitoring off therapy. To our knowledge, this is the first reported case of a complete pathologic response to GEMOX chemotherapy in the context of CRS/HIPEC for peritoneal metastases in HCC, after progression on standard immunotherapy and tyrosine kinase inhibitor treatments. In this report, we review the current systemic treatment landscape in HCC. We highlight potential consideration of cytotoxic chemotherapy, which is less frequently utilized in current practice, in selected patients with HCC, and discuss the role of CRS/HIPEC in the management of peritoneal metastases. Further investigation regarding predictors of response to systemic treatments is strongly needed. Multidisciplinary management may ultimately prolong survival in patients with advanced HCC.
Keywords: Hepatocellular carcinoma; Systemic therapy; Chemotherapy; Cytoreductive surgery; HIPEC
| Introduction | ▴Top |
Hepatocellular carcinoma (HCC) remains a predominant type of liver cancer and a leading cause of cancer-related death worldwide [1]. Risk factors for HCC include cirrhosis, viral hepatitis (B and C), alcohol consumption, metabolic syndrome including diabetes and obesity, aflatoxin exposure, and hereditary hemochromatosis [2-4]. Approximately, 50% of HCC cases are diagnosed at an advanced stage, and despite curative surgical resection for earlier stage cases, 70% of patients have disease recurrence [5, 6]. Advanced, metastatic HCC carries a poor prognosis with a 5-year survival rate of 2% [6]. Current standard systemic treatment approaches for advanced disease include combination immunotherapy strategies typically in the front-line setting, followed by anti-angiogenic tyrosine kinase inhibitors (TKI), though data are limited for optimal sequencing strategies [6, 7]. On the other hand, cytotoxic chemotherapies are less frequently utilized, due to the lack of clear survival benefit demonstrated in clinical studies and often poor tolerability in patients with concomitant cirrhosis. Consequently, chemotherapy has been omitted in treatment algorithms from several expert society guidelines, though may still be considered in some institutions in selected cases [8, 9].
In this report, we describe a patient with chronic hepatitis B and C coinfection-associated non-cirrhotic HCC, who demonstrated no response to frontline immunotherapy with atezolizumab/bevacizumab and subsequent TKI, lenvatinib. Given lack of response to standard therapies, she was treated with chemotherapy with gemcitabine/oxaliplatin (GEMOX) and exhibited an unusually profound improvement of her disease. She eventually underwent cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) to remove all visible macroscopic evidence of disease, with no viable tumor found on pathology. To our knowledge, this is the first reported complete pathologically confirmed response to GEMOX in HCC after progression on immunotherapy and TKI, in the context of CRS/HIPEC.
| Case Report | ▴Top |
A 38-year-old woman with history of chronic hepatitis B virus (CHB) with low viral titer (96 IU/mL) and newly diagnosed, untreated hepatitis C virus (HCV) presented to the hospital with sudden-onset, right upper quadrant, postprandial abdominal pain. On physical exam, she was noted to be tachycardic and had tenderness to palpation in the right upper and lower quadrants of her abdomen, without rebound or guarding. Initial laboratory analyses showed normal blood counts, creatinine, and liver function tests. She underwent a computed tomography (CT) scan of her chest/abdomen/pelvis, which showed two hepatic masses measuring 6.8 cm and 4.3 cm in segments 8 and 6, respectively, with areas of heterogeneous enhancement and washout and contained hemorrhagic rupture in the smaller lesion, both designated as Liver Imaging Reporting and Data System (LIRADS)-5, consistent with HCC. There was no evidence of distant metastases on imaging. Her alpha fetoprotein (AFP) was 27,102 ng/mL (Fig. 1). Given the diagnosis of HCC in the setting of CHB, she was started on tenofovir alafenamide.
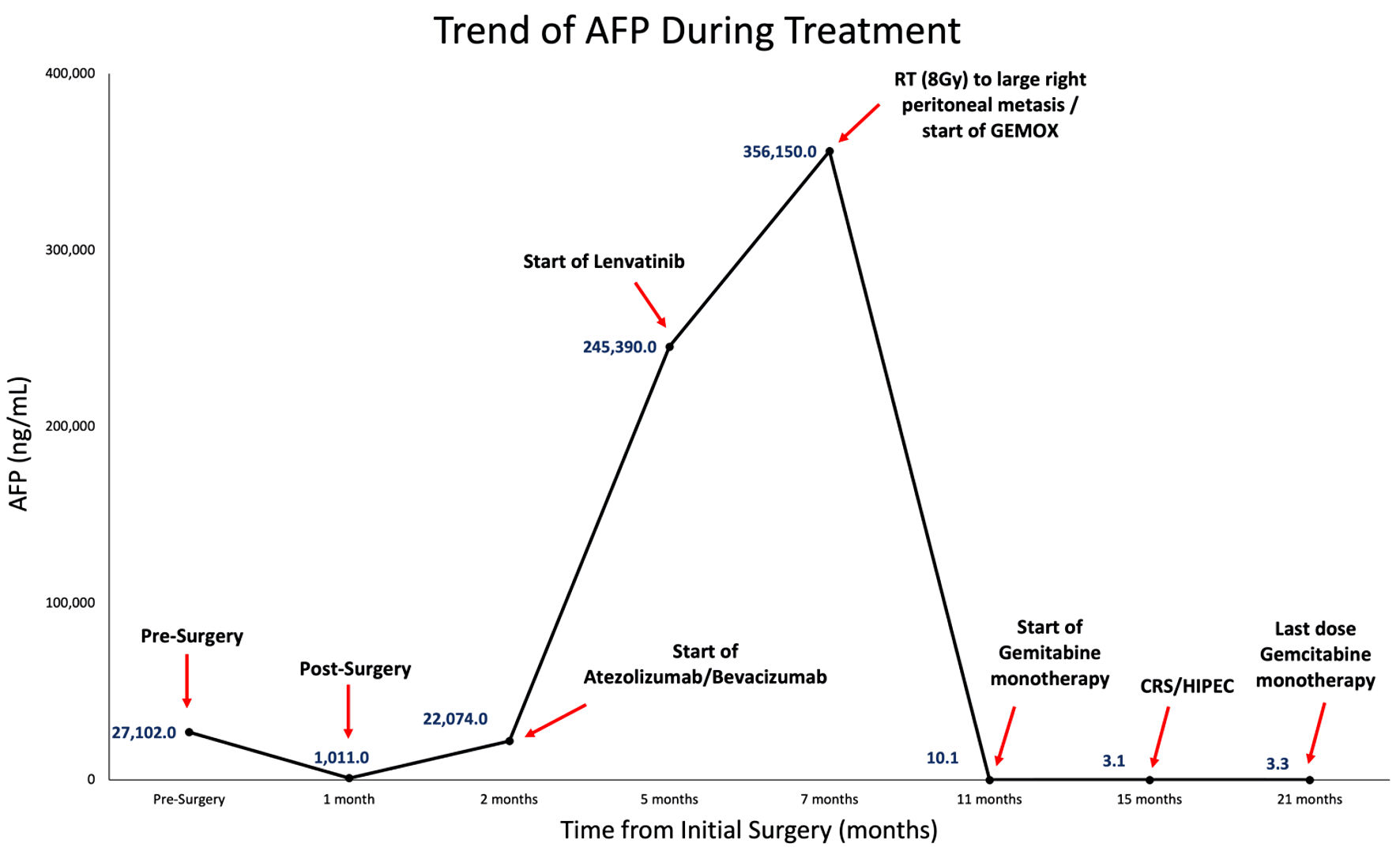 Click for large image | Figure 1. AFP trend starting from initial surgery to subsequent treatments (including atezolizumab/bevacizumab, lenvatinib, palliative radiation (RT) of 8 Gy to large right peritoneal metastasis, GEMOX, and gemcitabine monotherapy), CRS/HIPEC, and completion of postoperative gemcitabine therapy. GEMOX: gemcitabine and oxaliplatin; AFP: alpha fetoprotein; CRS: cytoreductive surgery; HIPEC: hyperthermic intraperitoneal chemotherapy. |
She underwent open right hepatectomy and intraoperatively the segment 6 lesion, which was necrotic appearing, notably ruptured during extraction. Pathology showed two foci (7 cm and 3.1 cm) of moderate to poorly differentiated HCC, with small and large vessel invasion present (Fig. 2a). Surgical margins were negative, and two lymph nodes which were excised were negative for carcinoma. Comprehensive genomic profiling showed that her tumor was microsatellite stable, with low tumor mutation burden (five mutations/megabase) and harbored a pathogenic mutation in KMT2D.
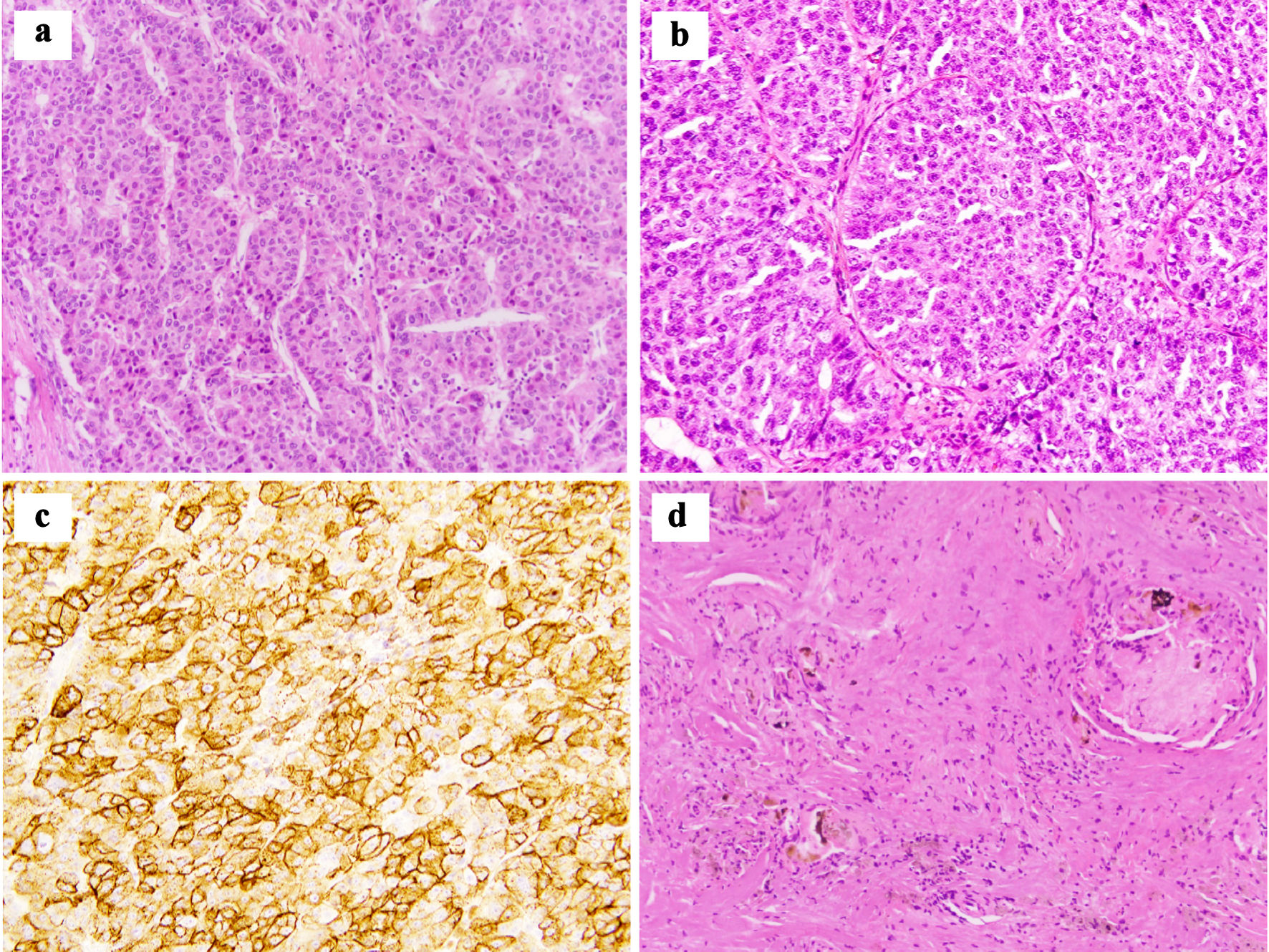 Click for large image | Figure 2. (a) Pathological analysis of initial tumor resection reveals moderately to poorly differentiated hepatocellular carcinoma with characteristic macrotrabecular growth pattern (hematoxylin and eosin, × 200). (b, c) Peritoneal nodule shows metastatic poorly differentiated hepatocellular carcinoma that stains diffusely positive for glypican 3 ((b) hematoxylin and eosin, × 200; (c) glypican 3, × 200). (d) Post-GEMOX resection specimen shows foci of necrosis and bile plugs surrounded by foreign body giant cell reaction with no viable tumor present (hematoxylin and eosin, × 200). GEMOX: gemcitabine and oxaliplatin. |
After surgery, her AFP initially reached a nadir of 1,011 ng/mL; however, at 6 weeks postoperatively it increased to 3,962 ng/mL (Fig. 1). She underwent a positron emission tomography-computed tomography (PET/CT) scan, which showed multiple new hypermetabolic peritoneal implants and hypermetabolic retroperitoneal lymph nodes (Fig. 3a). Given these new imaging findings with rising AFP, the overall clinical picture was consistent with rapid disease recurrence. She started treatment with atezolizumab and bevacizumab 2 months postoperatively. Given her untreated HCV, she received concurrent treatment with sofosbuvir/velpatasvir for 12 weeks with sustained virologic response and cure of HCV. After three cycles (2 months) of immunotherapy, repeat CT scans showed significant enlargement of peritoneal metastases (PMs), corresponding with rising AFP to 82,882 ng/mL. Her systemic treatment was then changed to lenvatinib; however, her AFP increased further to 357,321 ng/mL after a month (Fig. 1).
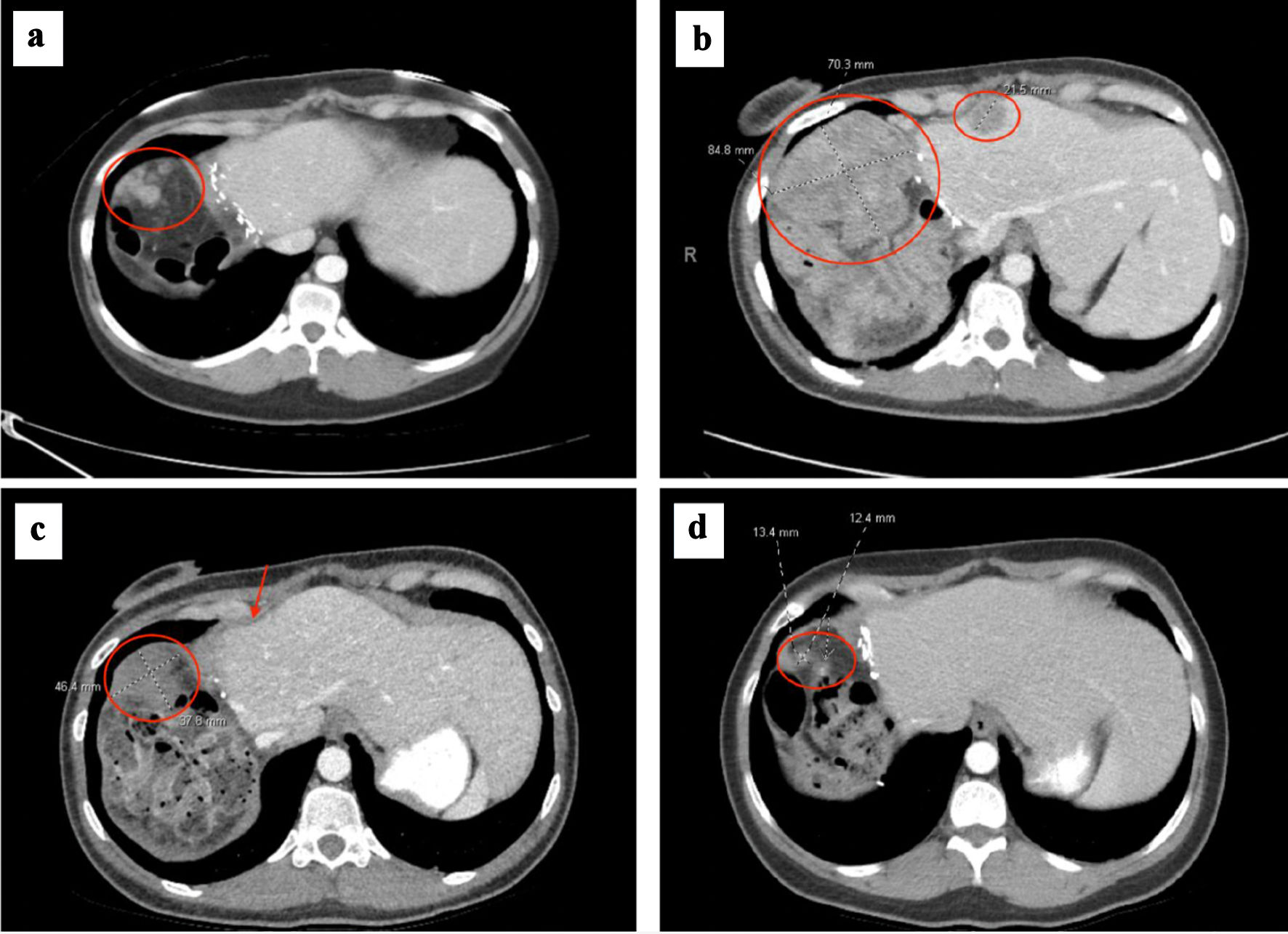 Click for large image | Figure 3. (a) Right upper quadrant peritoneal metastasis (circled) at time of metastatic recurrence after initial hepatectomy. (b) Right upper quadrant peritoneal metastasis (circled) increased to 8.5 cm, which had progressed after no response to atezolizumab/bevacizumab and lenvatinib. Smaller mid-anterior surface perihepatic implant also noted (circled). (c) Right upper quadrant peritoneal metastasis decreased to 4.6 cm (circled) and anterior perihepatic implant (arrow) resolved after 2 months of GEMOX. (d) Right upper quadrant peritoneal metastasis decreased to 1.3 cm prior to cytoreductive surgery and HIPEC, after several months of GEMOX followed by gemcitabine monotherapy. GEMOX: gemcitabine and oxaliplatin; HIPEC: hyperthermic intraperitoneal chemotherapy. |
Given lack of tumor control through systemic agents, she was evaluated by surgical oncology for consideration of CRS and HIPEC. She underwent a diagnostic laparoscopy (DL), and was noted to have bulky peritoneal disease, primarily in the right upper quadrant and epigastrium; however, her small bowel, mesentery, and pelvis were uninvolved (Fig. 2b, c). Her calculated peritoneal cancer index (PCI) was 10, which was scored and calculated as described by Jacquet et al [10]. Figure 2b, c demonstrates representative peritoneal biopsies taken during the DL that showed peritoneal metastasis consistent with poorly differentiated HCC.
Postoperatively, she developed worsened abdominal pain, nausea, and vomiting, requiring hospitalization for pain management. Repeat CT imaging demonstrated further disease progression with multiple PMs, with the largest lesion now 8.5 cm (Fig. 3b). Due to continued rapid progression without systemic control of disease, consideration of CRS/HIPEC was withheld. She received a single fraction (8 Gy) of palliative radiation to the large, right abdominal peritoneal metastasis for pain control. She was additionally treated with escalating doses of opioids for pain management. Given lack of response to immunotherapy and anti-angiogenic TKI therapy, and clinical presentation of painful, large tumor burden with preserved liver function, she was started on a cytotoxic chemotherapy with gemcitabine and oxaliplatin (GEMOX) administered every other week. She tolerated chemotherapy well, her abdominal pain began to improve, and she was subsequently discharged from the hospital.
She continued to receive GEMOX and exhibited a rapid decline in AFP to 2,010 ng/mL after 2 months of treatment. Interval CT imaging showed significant reduction in size of peritoneal lesions by nearly 50% (Fig. 3c). By 4 months of chemotherapy, her AFP normalized to 4.3 ng/mL. CT scans showed continued response, with the largest lesion now 3.4 cm. She was then transitioned to gemcitabine monotherapy given cumulative neurotoxicity from oxaliplatin, which included symptoms of peripheral neuropathy and foot drop, the latter of which improved after oxaliplatin was discontinued. Her peritoneal disease continued to improve on CT imaging after 3 more months of gemcitabine, and her AFP remained within normal range. Her cancer-associated pain improved and remained well-controlled with chemotherapy in addition to medical pain management.
With her remarkable response to chemotherapy and peritoneal-only disease on CT imaging and continued normalization of AFP, she underwent further multidisciplinary review. After extensive discussion of risks and benefits, the decision was to incorporate CRS and HIPEC in her disease management to optimize oncologic outcome. After an additional 2 months of gemcitabine monotherapy with continued disease control on imaging (Fig. 3d), she underwent a complete macroscopic tumor cytoreduction (score - CCR 0), which included removing the disease-bearing omentum, right upper quadrant parietal peritoneum, right and left anterior peritoneum, transverse mesocolon peritoneal implants, total abdominal hysterectomy, bilateral salpingo-oophorectomy, followed by HIPEC with mitomycin-C (MMC) and cisplatin for a calculated total PCI of 10. HIPEC with 40 mg of MMC was employed over the entire 90-min perfusion period that included 126 mg of cisplatin delivered during the latter 60 min.
Surgical pathology from all specimens was negative for any viable evidence of tumor and demonstrated fibroadipose tissue with necrosis, calcification, foamy histiocyte collection and foreign body giant cell reaction, consistent with a remarkable treatment effect (Fig. 2d). She tolerated the surgery well, and follow-up imaging 3 months postoperatively showed no evidence of recurrent disease. She then completed an additional 2 months of postoperative gemcitabine monotherapy, which was complicated by cumulative fatigue and thrombocytopenia requiring dose delays and reduction. Given treatment-related toxicities, no radiographic evidence of disease with normal AFP, and no clear standardized approach in this setting, the decision was to monitor closely off therapy, and resume chemotherapy if she developed future disease recurrence. To date, she has continued with ongoing surveillance.
| Discussion | ▴Top |
Presently, the recommended frontline treatment approach for advanced unresectable or metastatic HCC involves combination immunotherapy strategies, including atezolizumab (anti-programmed cell death ligand 1 (PD-L1) monoclonal antibody (mAb)) plus bevacizumab (anti-vascular endothelial growth factor (VEGF) mAb), or durvalumab (anti-PD-L1 mAb) plus tremelimumab (anti-cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) mAb). Overactivation of the VEGF signaling pathway, which is involved in tumor angiogenesis, results in recruitment of immunosuppressive cells and may also increase the expression of the programmed cell death 1 (PD-1) receptors on tumor-infiltrating T cells. PD-1 binding to the PD-L1 leads to T cell suppression. Therefore, combining VEGF and PD-1 inhibition may increase T cell activity and anti-tumor immunity [6, 11-13]. The pivotal IMbrave150 trial consequently confirmed the superiority of atezolizumab/bevacizumab over sorafenib (anti-VEGF TKI) for first-line treatment of advanced unresectable or metastatic HCC, with improvement in median overall survival (OS) (19.2 months vs. 13.4 months), progression-free survival (PFS) (6.8 months vs. 4.3 months), overall response rate (29.8% vs. 11.3%), and complete response rate (CRR) (7.7% vs. 0.6%), respectively [14]. Sub-group analyses of survival favored atezolizumab/bevacizumab in patients with viral hepatitis (B or C) but suggested possibly less benefit for patients with non-viral etiology. Follow-up real-world analysis of the combination however has not shown a clear association between viral vs. non-viral etiology and survival [15]. Although a well-tolerated regimen, immune-mediated hepatitis occurred in 53% of patients at severity level, and with more severe grade (3 or 4) occurring in 25% [13]. VEGF inhibition may be associated with specific adverse events, such as hypertension, proteinuria, thromboembolic events, and bleeding, particularly from the gastrointestinal tract [14, 16]. Patients receiving atezolizumab/bevacizumab also commonly experienced hypertension and proteinuria [13]. On the other hand, patients receiving sorafenib more commonly experienced diarrhea and hand-foot syndrome, which may more directly impact quality of life [13]. Moreover, patient-reported outcomes demonstrated that time to deterioration of quality of life was more favorable with atezolizumab/bevacizumab over sorafenib (11.2 months vs. 3.6 months, respectively) [17].
In patients with clinical contraindications such as high bleeding risk or recent cardiovascular event, a VEGF inhibitor-free regimen may be preferred in the frontline. Combinations of PD-1 and CTLA-4 inhibitors have demonstrated both significant anti-tumor and immunostimulatory effects in HCC [18, 19]. The phase III HIMALAYA trial established a single initial priming dose of tremelimumab plus monthly durvalumab as another frontline treatment option for advanced HCC, with improvement in median OS, compared with sorafenib in patients with unresectable HCC (16.4 months vs. 13.7 months, respectively) [19]. OS sub-group analyses showed greater benefit in patients with hepatitis B compared with hepatitis C; patients with non-viral etiology also derived significant improvement in survival. Though generally well tolerated, 50.5% of patients experienced treatment-related grade 3 or 4 adverse events, with 20.1% of patients experiencing immune-mediated adverse events requiring high dose steroids [20]. Alternatively, in patients who are ineligible for combination therapy with atezolizumab and bevacizumab or durvalumab and tremelimumab, durvalumab monotherapy may also be an acceptable alternative to sorafenib [19]. Quality of life assessments also demonstrated significant improvement in time to deterioration from disease-related symptoms of both durvalumab/tremelimumab and durvalumab alone, compared with sorafenib [21].
Notably, if there is a contraindication to frontline immunotherapy, other treatment options include anti-VEGF TKIs, such as sorafenib or lenvatinib, which inhibit multiple pathways critical for angiogenesis and cell proliferation [6, 22]. Sorafenib was the first systemic agent to show a survival benefit compared with placebo in advanced HCC, based on the SHARP trial [6, 23]. Subsequently, the phase III REFLECT study demonstrated non-inferiority of lenvatinib compared with sorafenib in terms of OS (13.6 months vs. 12.3 months, respectively), but superior objective response rate (40.6% vs. 12.4%), longer time to progression (TTP) (7.4 months vs. 3.7 months), and PFS (7.3 months vs. 3.6 months) [6, 24]. In terms of adverse events, patients receiving lenvatinib experienced higher incidence of hypertension compared with sorafenib, while patients receiving sorafenib had a higher incidence of hand-foot syndrome [24]. Time to clinically meaningful deterioration of certain quality of life parameters, such as pain and diarrhea, were observed sooner in the sorafenib arm compared with lenvatinib, however overall summary score was not significantly different between the two arms [24]. Given the current utilization of immune checkpoint inhibitors in the first-line setting for most patients, these agents may also currently be considered in the second-line setting, though data of efficacy beyond first-line are limited [25, 26].
Beyond initial immunotherapy and TKIs such as lenvatinib and sorafenib, other multikinase TKIs (regorafenib, cabozantinib), VEGF monoclonal antibodies (ramucirumab), and combination checkpoint inhibitors (nivolumab plus ipilimumab) have been studied and remain potential later-line treatment options [6, 27-31]. The CELESTIAL study noted significant improvement in OS of cabozantinib, a VEGF TKI which additionally targets MET and AXL, over best supportive care (10.2 months vs. 8.0 months, respectively), in sorafenib-experienced patients, some of whom received up to two prior lines of therapy [29]. In further subgroup analyses, those patients who received only prior sorafenib experienced a greater magnitude of survival benefit compared with placebo (11.3 months vs. 7.2 months, respectively) [32]. Adverse events common to VEGF TKIs were also observed with cabozantinib, including hand-foot syndrome, hypertension, elevated liver tests, fatigue, and diarrhea [29]. Although treatment was associated with initial reduction in health utility due to treatment-related side effects, it led to overall clinically meaningful increase in quality-adjusted life years compared with placebo, with quality-of-life assessment [33]. Nonetheless, optimal sequencing after first-line immunotherapy remains unclear, due to the lack of directly comparative data between second-line and beyond regimens, with most of these studies comparing treatment to best supportive care alone and requiring prior sorafenib, which is no longer the frontline standard.
Cytotoxic chemotherapy historically has been less commonly utilized in HCC, as it has not demonstrated clear OS benefit and is often difficult to tolerate in the setting of underlying liver dysfunction, such as cirrhosis. Increased drug resistance has also been reported in HCC tumor cells [4, 34, 35]. Nonetheless, for patients who may not be candidates for or who have progressed on prior immunotherapy or TKI, combination chemotherapy regimens may still be considered in the appropriate clinical setting, particularly in patients without decompensated liver cirrhosis. Oxaliplatin-based regimens combined with gemcitabine or a fluoropyrimidine, such as fluorouracil or capecitabine, have been investigated [4, 36], with variable reported efficacy outcomes. Utilization of GEMOX has been supported by phase II studies and retrospective cohort analyses. In a phase II trial by Louafi et al, previously untreated patients with advanced HCC received GEMOX, and were noted to have response rate of 18%, disease control rate of 76%, median PFS of 6.3 months and median OS of 11.5 months, with potentially greater efficacy in those with nonalcoholic cirrhosis than alcoholic cirrhosis [37]. In another phase II study by Lee et al, patients with advanced HCC who progressed on or could not tolerate sorafenib and received GEMOX demonstrated a median PFS of 3.9 months and median OS of 10.5 months, indicating potential efficacy in later-line treatment settings [38]. Additional retrospective cohort studies have also confirmed anti-cancer activity of GEMOX in patients both anti-angiogenic therapy naive and experienced [39, 40]. Nonetheless, this chemotherapy regimen carries higher risk of myelosuppression with risk of infection due to neutropenia, as well as dose-limiting neurotoxicity with risk of long-term, residual neuropathy which may impact quality of life. Our patient demonstrated progressive neuropathy leading to foot drop, requiring discontinuation of oxaliplatin after a few months of treatment, which improved after cessation. Therefore, despite the addition of numerous antineoplastic therapies in HCC such as immunotherapy and anti-angiogenic inhibitors, cytotoxic chemotherapy may still play a role in appropriately selected patients, particularly when they have failed typical standard therapies.
Most importantly, given the lack of response our patient demonstrated to standard lines of therapy, including immunotherapy and anti-VEGF TKI therapy, but an unusually deep response to cytotoxic chemotherapy, there remains a significant unmet need to identify biomarkers which may predict better response to specific types of therapies in HCC. Molecular characterization in HCC may be impacted by tumor genetic heterogeneity, as well as differing underlying disease etiologies. While certain molecular classifications have been demonstrated in some studies to have prognostic relevance, their predictive capacity is yet to be determined [41]. In a post-hoc analysis of the IMbrave 150 trial, Zhu et al identified molecular correlates associated with better clinical response to atezolizumab/bevacizumab including high expression of CD274, T-effector signature and intratumoral CD8+ T cell density, and high expression of VEGF receptor 2, T regulatory cells, and myeloid inflammatory signatures [42]. The ongoing, prospective NCI-CLARITY study (National Cancer Institute Cancers of the Liver: Accelerating Research of Immunotherapy by a Transdisciplinary Network) is exploring the mechanisms of immunotherapy response and resistance in patients with liver cancer receiving upfront immunotherapy through biospecimen collection and correlative laboratory analysis [43]. Thus, predictive biomarkers of response are urgently needed to help better select both upfront and sequential treatment options for patients with HCC.
CRS and HIPEC after initial chemotherapy have been investigated in other cancer types [44, 45], with more established roles in the setting of peritoneal involvement in certain malignancies, such as appendiceal/pseudomyxoma peritonei and ovarian cancer [46-49]. Notably, CRS/HIPEC has been studied in selected patients with HCC and PMs, with a demonstrable possible PFS benefit [50-52].
In the field of peritoneal surface malignancies, appropriate selection of patients with PMs who may benefit from CRS/HIPEC requires careful multidisciplinary review and consideration of specific clinicopathologic factors. The PCI, used to determine abdominopelvic peritoneal tumor burden, involves dividing the abdomen into 13 regions and scoring according to tumor size; this score (PCI range: 0 - 39) predicts likelihood of complete cytoreduction and correlates with survival [53]. In general, for more invasive, high-grade cancers, a PCI score of more than 20 typically precludes CRS/HIPEC, and it is not recommended, as the OS at 5 years approaches 0 [54]. Accurate calculation of PCI in our experience is largely determined in the operating room during either DL and/or laparotomy as described in this patient. Additional characteristics that may preclude CRS/HIPEC in part include tumor involvement at the root of the mesentery, hepatic pedicle, retroperitoneal extension, and invasive bladder involvement. Histopathologic assessment is also considered during patient selection; for example, noninvasive malignancies may respond more favorably to peritonectomy than invasive cancers [55]. Other clinical contraindications to CRS/HIPEC include severe cardiopulmonary disease, hepatic disease, and renal failure. In addition, preparation for HIPEC requires experienced centers with surgical and oncology expertise, experienced pharmacy and anesthesiology staff, and specific equipment, including a heat exchanger, outflow and inflow catheters, temperature probes, chemotherapy reservoir, and a computer system that controls the heat exchange. Typical cytotoxic chemotherapy used for HIPEC includes mitomycin C, cisplatin, doxorubicin, paclitaxel, irinotecan, and platinum agents. Our choice for doublet intraperitoneal (IP) regional therapy employing mitomycin C and cisplatin in this case utilized a potent alkylating agent and platinum compound protocoled with sodium thiosulfate, respectively; each agent providing a high molecular weight conducive to IP drug retention and an optimal therapeutic concentration time curve ratio with thermal temperature enhancement, tumor penetrance and treatment response [50-52, 56, 57].
Limited data exist regarding the utility of CRS/HIPEC in patients with HCC and PMs. Prognosis in this setting remains poor, with a median survival of 6 - 14 months [58]. However, in a retrospective analysis of 21 patients with HCC and peritoneal carcinomatosis (PC) treated with CRS/HIPEC at multiple centers, Mehta et al reported a median OS of 46.7 months, with 3-year and 5-year OS rates of 88.9% and 49.4%, respectively, and median recurrence-free survival of 26.3 months [52]. In this study, complete cytoreduction (achieved in 76% of patients) in addition to perioperative chemotherapy and a number of IP chemotherapeutic agents used during HIPEC were significant prognostic factors associated with survival. In a single-center evaluation of CRS and HIPEC in patients with HCC and PC, Tabrizian et al noted that in patients achieving complete cytoreduction, median OS was 35.6 months, and time to recurrence was 23 months [51]. Though most patients recurred in this cohort, patients achieved notably longer survival compared to historical outcomes with systemic therapy only. Furthermore, Berger et al reported on the impact of extrahepatic metastatectomy in selected patients with advanced HCC, noting a significant prolongation of OS in patients who received systemic treatment and underwent extrahepatic metastatectomy versus those who received systemic treatment alone (median OS 27.2 vs. 7.4 months, respectively) [58]. Hence, in carefully selected patients with HCC treated with systemic therapies, additional resection of metastatic disease may extend survival.
In this case, our patient’s tumor had no response to immunotherapy with atezolizumab/bevacizumab or anti-angiogenic TKIs such as lenvatinib. However, she subsequently demonstrated an atypical, deep response to GEMOX chemotherapy leading to complete cytoreduction and HIPEC of her peritoneal disease. Adding to this, she achieved a histologically confirmed complete response to cytotoxic chemotherapy, which is rare. Nonetheless, the underlying biological mechanism of this response is unclear, and was not explained by her genomic tumor profile. Further research is needed to better understand predictors of response to different types of therapy in HCC. Finally, we demonstrate the successful incorporation of CRS/HIPEC in her management after careful multidisciplinary review, to provide the best possibility of disease remission and improving long-term survival in this young patient. Limitations of this case report include the retrospective nature of this review and duration of follow-up. Furthermore, its applicability to a general population is currently limited, and an examination of larger cohorts of patients would be needed to validate this treatment strategy.
Ultimately, the question remains of whether “proof of concept” may be demonstrated in similar patients with advanced HCC and PM using the combination of preoperative GEMOX or other systemic therapy regimens with CRS/HIPEC, which may contribute to similar durable disease control. Our case suggests that it may. This report further adds to the existing body of literature supporting an aggressive treatment approach in these unique, highly selected patients with HCC. Given limited evidence for utilization of CRS and HIPEC in patients with HCC and PMs, performance of this procedure should be limited to very experienced, multidisciplinary high-volume centers.
Learning points
This case demonstrates that despite the progress in systemic treatments in HCC with immunotherapy and anti-angiogenic agents, there may still be a role for cytotoxic chemotherapy such as GEMOX in selected patients with HCC, particularly in those who have failed standard recommended therapies and remain with good performance status without liver decompensation. Appropriate selection of patients for metastatectomy, in this case, CRS/HIPEC for PMs, may palliate symptomatic tumor burden, and provide a chance at disease remission. Multidisciplinary management of these patients to explore potential treatment options is paramount to achieving the best possible survival outcomes in an otherwise morbid and deadly malignancy.
Acknowledgments
None to declare.
Financial Disclosure
The authors have no financial disclosure to report.
Conflict of Interest
The authors have no conflict of interest.
Informed Consent
Verbal informed consent was obtained from the patient for this case report.
Author Contributions
AM, SA, WB, and DL contributed to the drafting and writing of the manuscript. AM, JE, WJ, and DL contributed to the production of the figures. All authors contributed to the visualization and editing of the manuscript.
Data Availability
The data used in this report were cited within the manuscript. Further inquiries should be directed to the corresponding author.
Abbreviations
HCC: hepatocellular carcinoma; GEMOX: gemcitabine and oxaliplatin; AFP: alpha fetoprotein; CRS: cytoreductive surgery; HIPEC: hyperthermic intraperitoneal chemotherapy; IP: intraperitoneal; TKI: tyrosine kinase inhibitor; CHB: chronic hepatitis B virus; HCV: hepatitis C virus; CT: computed tomography; PCI: peritoneal cancer index; mAb: monoclonal antibody; VEGF: vascular endothelial growth factor; PD-1: programmed cell death 1; PD-L1: programmed cell death ligand 1; OS: overall survival; PFS: progression-free survival; CRR: complete response rate; TTP: time to progression; PM: peritoneal metastases
| References | ▴Top |
- Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - McGlynn KA, Petrick JL, El-Serag HB. Epidemiology of hepatocellular carcinoma. Hepatology. 2021;73(Suppl 1):4-13.
doi pubmed pmc - Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin. 2021;71(1):7-33.
doi pubmed - Fan Y, Xue H, Zheng H. Systemic therapy for hepatocellular carcinoma: current updates and outlook. J Hepatocell Carcinoma. 2022;9:233-263.
doi pubmed pmc - Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg. 2015;261(5):947-955.
doi pubmed - Leowattana W, Leowattana T, Leowattana P. Systemic treatment for unresectable hepatocellular carcinoma. World J Gastroenterol. 2023;29(10):1551-1568.
doi pubmed pmc - Caturano A, Monda M, Galiero R, Vetrano E, Giorgione C, Mormone A, Rinaldi M, et al. Current hepatocellular carcinoma systemic pharmacological treatment options. World Cancer Res J. 2023;10:e2570.
doi - Vogel A, Martinelli E, ESMO Guidelines Committee. Updated treatment recommendations for hepatocellular carcinoma (HCC) from the ESMO Clinical Practice Guidelines. Ann Oncol. 2021;32(6):801-805.
doi pubmed - NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®). Hepatocellular Carcinoma. Available online: https://www.nccn.org/professionals/physician_gls/pdf/hcc.pdf (accessed on January 8).
- Jacquet P, Sugarbaker PH. Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res. 1996;82:359-374.
doi pubmed - Dong Y, Wong JSL, Sugimura R, Lam KO, Li B, Kwok GGW, Leung R, et al. Recent advances and future prospects in immune checkpoint (ICI)-based combination therapy for advanced HCC. Cancers (Basel). 2021;13(8):1949.
doi pubmed pmc - Chen Y, Hu H, Yuan X, Fan X, Zhang C. Advances in immune checkpoint inhibitors for advanced hepatocellular carcinoma. Front Immunol. 2022;13:896752.
doi pubmed pmc - Cheng AL, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Lim HY, et al. Updated efficacy and safety data from IMbrave150: Atezolizumab plus bevacizumab vs. sorafenib for unresectable hepatocellular carcinoma. J Hepatol. 2022;76(4):862-873.
doi pubmed - Finn RS, Qin S, Ikeda M, Galle PR, Ducreux M, Kim TY, Kudo M, et al. Atezolizumab plus bevacizumab in unresectable hepatocellular carcinoma. N Engl J Med. 2020;382(20):1894-1905.
doi pubmed - Brown TJ, Mamtani R, Gimotty PA, Karasic TB, Yang YX. Outcomes of hepatocellular carcinoma by etiology with first-line atezolizumab and bevacizumab: a real-world analysis. J Cancer Res Clin Oncol. 2023;149(6):2345-2354.
doi pubmed pmc - Hatanaka T, Naganuma A, Yata Y, Kakizaki S. Atezolizumab plus bevacizumab and tremelimumab plus durvalumab: how should we choose these two immunotherapy regimens for advanced hepatocellular carcinoma? Hepatobiliary Surg Nutr. 2022;11(6):927-930.
doi pubmed pmc - Galle PR, Finn RS, Qin S, Ikeda M, Zhu AX, Kim TY, Kudo M, et al. Patient-reported outcomes (PROs) from the Phase III IMbrave150 trial of atezolizumab (atezo) + bevacizumab (bev) vs sorafenib (sor) as first-line treatment (tx) for patients (pts) with unresectable hepatocellular carcinoma (HCC). Journal of Clinical Oncology. 2020;38:476.
doi - Kudo M. Scientific rationale for combination immunotherapy of hepatocellular carcinoma with anti-PD-1/PD-L1 and anti-CTLA-4 antibodies. Liver Cancer. 2019;8(6):413-426.
doi pubmed pmc - Kudo M. Durvalumab plus tremelimumab in unresectable hepatocellular carcinoma. Hepatobiliary Surg Nutr. 2022;11(4):592-596.
doi pubmed pmc - Abou-Alfa GK, Lau G, Kudo M, Chan SL, Kelley RK, Furuse J, Sukeepaisarnjaroen W, et al. Tremelimumab plus Durvalumab in Unresectable Hepatocellular Carcinoma. NEJM Evid. 2022;1(8):EVIDoa2100070.
doi pubmed - Sangro B, Galle PR, Kelley RK, Charoentum C, Toni END, Ostapenko Y, Heo J, et al. Patient-reported outcomes from the phase 3 HIMALAYA study of tremelimumab plus durvalumab in unresectable hepatocellular carcinoma. Journal of Clinical Oncology. 2022;40:4074.
doi - Ranieri G, Gadaleta-Caldarola G, Goffredo V, Patruno R, Mangia A, Rizzo A, Sciorsci RL, et al. Sorafenib (BAY 43-9006) in hepatocellular carcinoma patients: from discovery to clinical development. Curr Med Chem. 2012;19(7):938-944.
doi pubmed - Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378-390.
doi pubmed - Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391(10126):1163-1173.
doi pubmed - Palmer ME, Gile JJ, Storandt MH, Jin Z, Zemla TJ, Tran NH, Mahipal A. Outcomes of Patients with Advanced Hepatocellular Carcinoma Receiving Lenvatinib following Immunotherapy: A Real World Evidence Study. Cancers (Basel). 2023;15(19):4867.
doi pubmed pmc - Qin HN, Ning Z, Sun R, Jin CX, Guo X, Wang AM, Liu JW. Lenvatinib as second-line treatment in patients with unresectable hepatocellular carcinoma: A retrospective analysis. Front Oncol. 2022;12:1003426.
doi pubmed pmc - Zhu AX, Finn RS, Mulcahy M, Gurtler J, Sun W, Schwartz JD, Dalal RP, et al. A phase II and biomarker study of ramucirumab, a human monoclonal antibody targeting the VEGF receptor-2, as first-line monotherapy in patients with advanced hepatocellular cancer. Clin Cancer Res. 2013;19(23):6614-6623.
doi pubmed pmc - Bruix J, Qin S, Merle P, Granito A, Huang YH, Bodoky G, Pracht M, et al. Regorafenib for patients with hepatocellular carcinoma who progressed on sorafenib treatment (RESORCE): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2017;389(10064):56-66.
doi pubmed - Abou-Alfa GK, Meyer T, Cheng AL, El-Khoueiry AB, Rimassa L, Ryoo BY, Cicin I, et al. Cabozantinib in patients with advanced and progressing hepatocellular carcinoma. N Engl J Med. 2018;379(1):54-63.
doi pubmed pmc - Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D, Verslype C, et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol. 2018;19(7):940-952.
doi pubmed - Yau T, Kang YK, Kim TY, El-Khoueiry AB, Santoro A, Sangro B, Melero I, et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol. 2020;6(11):e204564.
doi pubmed pmc - Kelley RK, Ryoo BY, Merle P, Park JW, Bolondi L, Chan SL, Lim HY, et al. Second-line cabozantinib after sorafenib treatment for advanced hepatocellular carcinoma: a subgroup analysis of the phase 3 CELESTIAL trial. ESMO Open. 2020;5(4):E000714.
doi pubmed pmc - Freemantle N, Mollon P, Meyer T, Cheng AL, El-Khoueiry AB, Kelley RK, Baron AD, et al. Quality of life assessment of cabozantinib in patients with advanced hepatocellular carcinoma in the CELESTIAL trial. Eur J Cancer. 2022;168:91-98.
doi pubmed - Huang CC, Wu MC, Xu GW, Li DZ, Cheng H, Tu ZX, Jiang HQ, et al. Overexpression of the MDR1 gene and P-glycoprotein in human hepatocellular carcinoma. J Natl Cancer Inst. 1992;84(4):262-264.
doi pubmed - Ng IO, Liu CL, Fan ST, Ng M. Expression of P-glycoprotein in hepatocellular carcinoma. A determinant of chemotherapy response. Am J Clin Pathol. 2000;113(3):355-363.
doi pubmed - Qin S, Bai Y, Lim HY, Thongprasert S, Chao Y, Fan J, Yang TS, et al. Randomized, multicenter, open-label study of oxaliplatin plus fluorouracil/leucovorin versus doxorubicin as palliative chemotherapy in patients with advanced hepatocellular carcinoma from Asia. J Clin Oncol. 2013;31(28):3501-3508.
doi pubmed - Louafi S, Boige V, Ducreux M, Bonyhay L, Mansourbakht T, de Baere T, Asnacios A, et al. Gemcitabine plus oxaliplatin (GEMOX) in patients with advanced hepatocellular carcinoma (HCC): results of a phase II study. Cancer. 2007;109(7):1384-1390.
doi pubmed - Lee CK, Kim HS, Choi HJ. Phase II study of gemcitabine with oxaliplatin (GEMOX) in heavily treated patients with advanced hepatocellular carcinoma after failure of sorafenib treatment (PEACH). Journal of Clinical Oncology. 2022;40:456.
doi - Patrikidou A, Sinapi I, Regnault H, Fayard F, Bouattour M, Fartoux L, Faivre S, et al. Gemcitabine and oxaliplatin chemotherapy for advanced hepatocellular carcinoma after failure of anti-angiogenic therapies. Invest New Drugs. 2014;32(5):1028-1035.
doi pubmed - Zaanan A, Williet N, Hebbar M, Dabakuyo TS, Fartoux L, Mansourbakht T, Dubreuil O, et al. Gemcitabine plus oxaliplatin in advanced hepatocellular carcinoma: a large multicenter AGEO study. J Hepatol. 2013;58(1):81-88.
doi pubmed - Vogel A, Meyer T, Sapisochin G, Salem R, Saborowski A. Hepatocellular carcinoma. Lancet. 2022;400(10360):1345-1362.
doi pubmed - Zhu AX, Abbas AR, de Galarreta MR, Guan Y, Lu S, Koeppen H, Zhang W, et al. Molecular correlates of clinical response and resistance to atezolizumab in combination with bevacizumab in advanced hepatocellular carcinoma. Nat Med. 2022;28(8):1599-1611.
doi pubmed - National Translational Science Network of Precision-based Immunotherapy for Primary Liver Cancer. Available online: https://clinicaltrials.gov/study/NCT04145141 (accessed on April 29).
- Verwaal VJ, van Ruth S, de Bree E, van Sloothen GW, van Tinteren H, Boot H, Zoetmulder FA. Randomized trial of cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy and palliative surgery in patients with peritoneal carcinomatosis of colorectal cancer. J Clin Oncol. 2003;21(20):3737-3743.
doi pubmed - Seshadri RA, Glehen O. Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in gastric cancer. World J Gastroenterol. 2016;22(3):1114-1130.
doi pubmed pmc - van Driel WJ, Koole SN, Sikorska K, Schagen van Leeuwen JH, Schreuder HWR, Hermans RHM, de Hingh I, et al. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med. 2018;378(3):230-240.
doi pubmed - Lim MC, Chang SJ, Park B, Yoo HJ, Yoo CW, Nam BH, Park SY, et al. Survival After Hyperthermic Intraperitoneal Chemotherapy and Primary or Interval Cytoreductive Surgery in Ovarian Cancer: A Randomized Clinical Trial. JAMA Surg. 2022;157(5):374-383.
doi pubmed pmc - Huo YR, Richards A, Liauw W, Morris DL. Hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery (CRS) in ovarian cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2015;41(12):1578-1589.
doi pubmed - Chua TC, Moran BJ, Sugarbaker PH, Levine EA, Glehen O, Gilly FN, Baratti D, et al. Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol. 2012;30(20):2449-2456.
doi pubmed - Ji ZH, An SL, Li XB, Liu G, Li Y. Long-term progression-free survival of hepatocellular carcinoma with synchronous diffuse peritoneal metastasis treated by CRS+HIPEC: A case report and literature review. Medicine (Baltimore). 2019;98(8):e14628.
doi pubmed pmc - Tabrizian P, Franssen B, Jibara G, Sweeney R, Sarpel U, Schwartz M, Labow D. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy in patients with peritoneal hepatocellular carcinoma. J Surg Oncol. 2014;110(7):786-790.
doi pubmed - Mehta S, Schwarz L, Spiliotis J, Hsieh MC, Akaishi EH, Goere D, Sugarbaker PH, et al. Is there an oncological interest in the combination of CRS/HIPEC for peritoneal carcinomatosis of HCC? Results of a multicenter international study. Eur J Surg Oncol. 2018;44(11):1786-1792.
doi pubmed - Lambert LA. Looking up: Recent advances in understanding and treating peritoneal carcinomatosis. CA Cancer J Clin. 2015;65(4):284-298.
doi pubmed - da Silva RG, Sugarbaker PH. Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg. 2006;203(6):878-886.
doi pubmed - Ronnett BM, Yan H, Kurman RJ, Shmookler BM, Wu L, Sugarbaker PH. Patients with pseudomyxoma peritonei associated with disseminated peritoneal adenomucinosis have a significantly more favorable prognosis than patients with peritoneal mucinous carcinomatosis. Cancer. 2001;92(1):85-91.
doi pubmed - Ceelen WP, Flessner MF. Intraperitoneal therapy for peritoneal tumors: biophysics and clinical evidence. Nat Rev Clin Oncol. 2010;7(2):108-115.
doi pubmed - Van der Speeten K, Stuart OA, Chang D, Mahteme H, Sugarbaker PH. Changes induced by surgical and clinical factors in the pharmacology of intraperitoneal mitomycin C in 145 patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol. 2011;68(1):147-156.
doi pubmed - Kow AW, Kwon CH, Song S, Kim JM, Joh JW. Clinicopathological factors and long-term outcome comparing between lung and peritoneal metastasectomy after hepatectomy for hepatocellular carcinoma in a tertiary institution. Surgery. 2015;157(4):645-653.
doi pubmed - Berger Y, Spivack JH, Heskel M, Aycart SN, Labow DM, Sarpel U. Extrahepatic metastasectomy for hepatocellular carcinoma: Predictors of long-term survival. J Surg Oncol. 2016;114(4):469-474.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


