| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 000, Number 000, October 2024, pages 000-000
The Association of Preoperative Prognostic Nutritional Index With Survival Outcome in Ovarian Clear Cell Cancer
Nisa Prueksaritanonda, b, c , Kittisak Petchsilaa, Putsarat Insina, b
aGynecologic Oncology Unit, Department of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand
bCollege of Medicine, Rangsit University, Bangkok, Thailand
cCorresponding Author: Nisa Prueksaritanond, Department of Obstetrics and Gynecology, Rajavithi Hospital, Bangkok, Thailand
Manuscript submitted August 30, 2024, accepted October 8, 2024, published online October 30, 2024
Short title: PPNI and Ovarian Clear Cell Cancer Mortality
doi: https://doi.org/10.14740/wjon1963
| Abstract | ▴Top |
Background: The preoperative prognostic nutritional index (PPNI) has been investigated as a prognostic indicator in various cancers including epithelial ovarian cancer (EOC). However, its prognostic relevance in epithelial ovarian clear cell cancer (EOC-CC) remains uncertain. The objective of the study was to clarify the prognostic values of PPNI in EOC-CC patients.
Methods: We retrospectively reviewed 290 EOC-CC patients who underwent staging surgery at Rajavithi Hospital between January 2008 and December 2019. The PPNI was calculated using serum albumin × 10 (g/L) + 0.005 × peripheral blood lymphocyte count (per mm3). The association between PPNI and survival outcome was analyzed using the Kaplan-Meier method and the Cox proportional hazard model.
Results: The optimal cut-off value of PPNI, set at a mean of PPNI as 50, divided the EOC-CC patients into two groups: the low (n = 115) and the high (n = 175) PPNI group. With a median follow-up time of 63 months, patients with high PPNI exhibited significantly superior 5-year overall survival (OS) rates (76.4% vs. 56.8%, P = 0.004) and 5-year progression-free survival (PFS) rates (71.0% vs. 58.7%, P = 0.017) compared to patients with low PPNI. Univariate analysis revealed high PPNI correlated with increased OS (hazard ratio (HR): 0.51; 95% confidence interval (CI): 0.35 - 0.75) and PFS (HR: 0.63; 95% CI: 0.43 - 0.92). Nevertheless, in a multivariate analysis, high PPNI did not retain its status as an independent prognostic factor for a favorable prognosis in EOC-CC patients.
Conclusion: The present study did not confirm the prognostic significance of PPNI on survival outcomes in EOC-CC patients. Therefore, conducting prospective clinical research with large samples is necessary to illustrate the predictive values of PPNI in this rare disease.
Keywords: Epithelial ovarian cancer; Ovarian clear cell cancer; Prognostic nutritional index; Overall survival; Progression-free survival
| Introduction | ▴Top |
Ovarian cancer (OC) is one of the most common gynecologic malignancies worldwide, with the highest mortality rate. Epithelial ovarian cancer (EOC) constitutes the most common OC type and is categorized into five main histologic subtypes: high-grade serous, endometrioid, clear cell, mucinous, and low-grade serous carcinomas [1]. Among these, epithelial ovarian clear cell cancer (EOC-CC) represents the second most common histologic subtype, constituting approximately 10% of all EOC cases [1]. Its incidence varies from 5% to 25%, depending on geographic area and ethnicity. While EOC-CC represents 10% of cases in North America and Western countries, its incidence appears notably higher in Asia, accounting for 15% to 25% of EOC cases [2, 3]. At our institution, Rajavithi Hospital, Bangkok, Thailand, we have also observed a similar increasing trend in EOC-CC incidence, and it is now reaching up to 27%.
EOC-CC exhibited unique molecular pathogenesis, aggressive behavior, and inherent chemoresistance distinct from other EOC subtypes. Patients with EOC-CC are typically diagnosed at a younger age and earlier stage, with a 5-year disease-specific survival rate of 85.3% and 86.4% for stage I, similar to serous EOC [4, 5]. However, advanced-stage EOC-CC carries a significantly worse prognosis than serous EOC, with a higher hazard ratio (HR) for death (1.71; 95% confidence interval (CI): 1.57 - 1.86) in a meta-analysis [5, 6]. Additionally, EOC-CC responds less favorably to platinum-based chemotherapy compared to EOC subtypes [7]. Identifying prognostic factors for EOC-CC is essential for risk stratification, outcome prediction, and optimizing treatment strategies in this clinical context.
Even though serum cancer antigen 125 (CA125) is a well-established predictive marker for EOC, its prognostic relevance in EOC-CC remains uncertain. Prior research indicates lower CA125 levels in EOC-CC compared to other subtypes, with no clear correlation to clinical outcomes [8]. Investigating nutritional factors as predictors of clinical outcomes in EOC-CC and other cancers is gaining traction due to their association with malnutrition and systemic inflammation, which increase postoperative morbidity and poor clinical outcomes [9]. EOC patients, particularly those in an advanced stage, are at high risk of malnutrition due to cachexia and ascites. Systemic inflammation is a key driver of cancer initiation, progression, metastasis, and chemoresistance [10]. Thus, assessing the nutritional status and systemic inflammatory mediators is essential for predicting the prognosis of EOC.
The preoperative prognostic nutritional index (PPNI) was developed in 1984 to assess postoperative complications and derived from serum albumin and peripheral blood lymphocyte count [11]. Serum albumin is a recognized marker for nutritional status and independently predicts outcomes in various cancers, including EOC patients undergoing primary cytoreductive surgery [12]. Preoperative lymphopenia adversely affects progression-free survival (PFS) and overall survival (OS) in advanced-stage EOC patients [13]. Therefore, PPNI reflects nutritional and immunologic status and is a prognostic indicator for poor outcomes in various solid cancers, including EOC [14-17]. A previous meta-analysis of 2,050 OC patients demonstrated low PPNI correlated with shorter OS, PFS, and adverse clinicopathological parameters [18]. However, the rarity of EOC-CC limited the prognostic understanding. Also, in a recent study of 82 patients with early-stage EOC-CC patients, higher PPNI predicted a favorable prognosis, but there was no significant difference in recurrence-free survival [19]. To overcome these limitations, our study aimed to clarify the association between PPNI and predictive values in EOC-CC patients.
| Materials and Methods | ▴Top |
This retrospective cohort study comprises patients diagnosed with EOC-CC who were scheduled for cytoreductive surgery at Rajavithi Hospital from January 1, 2008 to December 31, 2019. The cohort was designed to assess the association between PPNI and survival outcomes in EOC-CC patients, with the primary endpoint being the 5-year OS of EOC-CC patients stratified by PPNI and the secondary endpoint being the 5-year PFS among the same stratified groups.
After receiving approval from the Institutional Review Board (IRB) of Rajavithi Hospital, all patients with EOC-CC diagnosed during the study period were identified from the Rajvithi Hospital’s cancer registry. Exclusion criteria included synchronous cancer patients, a history of prior cancer treated by chemotherapy or radiotherapy, mixed histopathological subtypes of EOC, and incomplete medical records. Additionally, patients with advanced-stage diseases who received neoadjuvant chemotherapy instead of primary cytoreductive surgery were excluded due to potential impacts on serum albumin and lymphocyte count.
All eligible patients underwent primary cytoreductive surgery, and tumor staging was determined according to the revised 2014 International Federation of Gynecology and Obstetrics (FIGO) staging system [20]. Adjuvant chemotherapy began within 4 weeks after surgery and continued for 3 - 6 cycles. Follow-up with pelvic examination and tumor markers assessment was conducted by gynecologic oncologists. Imaging studies (chest X-ray (CXR) and/or computed tomography (CT) scan) were performed if any suspicious lesion of recurrence or rising tumor markers were present.
Clinical data, including baseline characteristics (e.g., age at cancer diagnosis, body mass index (BMI) in kg/m2, and other comorbidities), preoperative laboratory studies (i.e., total white blood cell count, total lymphocyte count, hemoglobin level, platelet count, creatinine level, liver function test, and serum albumin), and serum CA125, which collected within 4 weeks before surgery, were obtained from the medical records. PPNI was determined using Onodera’s formula: serum albumin × 10 (g/L) + 0.005 × total lymphocyte count (per mm3) in the peripheral blood [12]. The PPNI cut-off value was defined as the mean of PPNI categorizing EOC-CC patients into low and high PPNI groups. Surgical and treatment details (e.g., operation date, operative findings, optimal surgery, residual tumor, staging, adjuvant treatment details, and each treatment’s date) were retrieved from the inpatient chart and tumor files.
Follow-up data were extracted from medical records, including the recurrence date, site of recurrence, and last visit. Dates and causes of death were obtained from the civil registration databases. Recurrence was histologically or clinically confirmed through imaging or increased serum marker levels and classified as locoregional recurrence (e.g., involving pelvic and nearby structures) or distant (e.g., abdominal organs, peritoneal carcinomatosis, liver, or bone). The recurrence date was defined as when the imaging study was performed, the blood sample for serum marker measurement was drawn, or the date of histopathological confirmation.
Sample size calculation
Sample size calculation followed the formula for testing two independent proportions using data from Yoshikawa et al’s study, where the 5-year OS was 97.6% for high PPNI and 76.8% for low PPNI [19, 21]. With a statistical power of 80% and a type I error 0.05, the required sample size was 40 subjects per group. Accounting for a 20% dropout rate, a minimum of at least 48 EOC-CC patients per group was needed.
Statistical analysis
Categorical variables comparing EOC-CC patients with high and low PPNI were analyzed using the Chi-square or Fisher’s exact test, with results presented as frequencies and percentages. For continuous data, the Student’s t-test was applied to normally distributed data (mean ± standard deviation) and the Mann-Whitney U test to non-normally distributed data (median and range). Kaplan-Meier method estimated OS and PFS curves, stratified by PPNI (high vs. low). OS was defined as the period from the date of operation until the date of death or the date of the last follow-up for the patients still alive at the end of the study. PFS was determined as the time elapsed from the operation date until the recurrence or death from any cause. The log-rank test compared OS and PFS between high and low PPNI groups. Multivariate Cox proportional hazard models assessed the association between PPNI and survival outcomes, considering covariates with a P-value less than 0.10 from the univariate analysis. HRs and 95% CIs were reported for the entire cohort. All statistical analyses were conducted using the Stata software package, version 15.1 (Stata Corp, College Station, Texas, USA), with the null hypothesis rejected if P-values were less than 0.05 or if the 95% CI did not include 1.
| Results | ▴Top |
From January 1, 2008 to December 31, 2019, a total of 290 EOC-CC patients who underwent primary staging surgery at Rajavithi Hospital were identified. Baseline clinical and clinicopathological characteristics of patients with EOC-CC stratified by PPNI are summarized in Table 1. In the study cohort, patients had a mean age of 53.3 ± 9.4 years, with 65.2% being post-menopausal. Mean BMI was 23.6 ± 4.6 kg/m2, and 65% had a BMI of less than 25 kg/m2. Approximately 20% of the patients had underlying medical conditions, with the most prevalent being hypertension (13.4%) and diabetic mellitus (6.2%).
 Click to view | Table 1. Baseline Clinical Characteristics of Patients With EOC-CC Stratified by PPNI |
Regarding laboratory findings, 75% had serum CA125 level ≥ 35 U/mL. Median white blood cell count was 7,635 cells/mm3 (range 3,000 - 16,500 cells/mm3), and median lymphocyte count was 26.7% (range 3.9-65.0%). Patients had an average serum albumin level of 4.1 ± 0.5 g/dL, indicating normal nutritional status. In addition, the mean hemoglobin level was 11.1 ± 1.4 g/dL, suggesting that the majority of patients experienced anemia.
According to clinicopathological characteristics, most patients (76.6%) were diagnosed with early-stage EOC (66.2% for stage I and 10.4% for stage II). More than two-thirds of patients (77.9%) underwent complete surgical staging, and nearly all (90.3%) achieved optimal surgery with no residual disease. Adjuvant chemotherapy was administered to 99% of patients, with 94.1% receiving paclitaxel and carboplatin over three cycles.
We calculated the mean PPNI from eligible patients as 50.7 ± 7.4 and set a cut-off value at 50 to categorize EOC-CC patients into the low PPNI (n = 115) and high PPNI groups (n = 175). The high PPNI group had a slightly higher proportion of obese patients than the low PPNI group (39.4% vs. 27.8%, P = 0.042). Conversely, the low PPNI group had more patients with serum CA125 levels ≥ 35 U/mL (84.4% vs. 69.1%, P = 0.003). Moreover, patients in the low PPNI group had a significantly lower hemoglobin level (10.7 ± 1.5 g/dL vs. 11.4 ± 1.4 g/dL, P < 0.001) and higher platelet count (395.2 ± 163.5 × 103 cells/mm3 vs. 333.6 ± 114.2 × 103 cells/mm3, P < 0.001). Regarding albumin levels and total lymphocyte count, the low PPNI group had significantly lower serum albumin levels (3.6 ± 0.6 g/dL vs. 4.4 ± 2.8 g/dL, P < 0.001) and a lower lymphocyte percentage (19.0% (range, 3.5 - 72.0) vs. 30.6% (range 12.7 - 63.7), P < 0.001).
In terms of surgical and clinicopathological characteristics, the high PPNI group had higher rates of complete surgical staging and optimal surgery (P < 0.05). In contrast, the low PPNI group had more frequent positive cytology of ascites (36.5% vs. 13.7%, P < 0.001). By stage distribution, patients in the high PPNI group presented at earlier stages than the low PPNI group (85.1% vs. 65.6%, P < 0.001). However, other baseline clinical data and clinicopathological characteristics were similar between the two groups.
Table 2 presents the pattern of recurrence and death in the EOC-CC patients stratified by the PPNI. Median follow-up was 63 months (interquartile range (IQR), 4 - 76 months). Among 290 patients, 109 (37.6%) had died by the time of the study’s end (December 31, 2021), with a higher mortality rate in the low PPNI group (49.6% vs. 29.7%, P < 0.001). However, most deaths were cancer-related, with similar rates in both groups (P = 0.192). Among 187 patients (64.5%) who remained alive without the disease at the end of the follow-up period, the low PPNI group had a higher recurrence rate than the high PPNI group (42.6% vs. 30.9%, P = 0.041). The most common site of recurrence was locoregional, with a comparable rate between both groups (P = 0.089). Based on the survival outcomes, our study found a 5-year OS rate of 68.6% (95% CI: 62.8-73.7%) and a 5-year PFS rate of 66.1% (95% CI: 60.3-71.3%) for EOC-CC patients. Kaplan-Meier curves (Fig. 1) indicated significantly shorter OS and PFS in those with low PPNI compared to high PPNI (log-rank test P-value < 0.05).
 Click to view | Table 2. Patterns of Recurrence and Death Stratified by PPNI in Patients With EOC-CC |
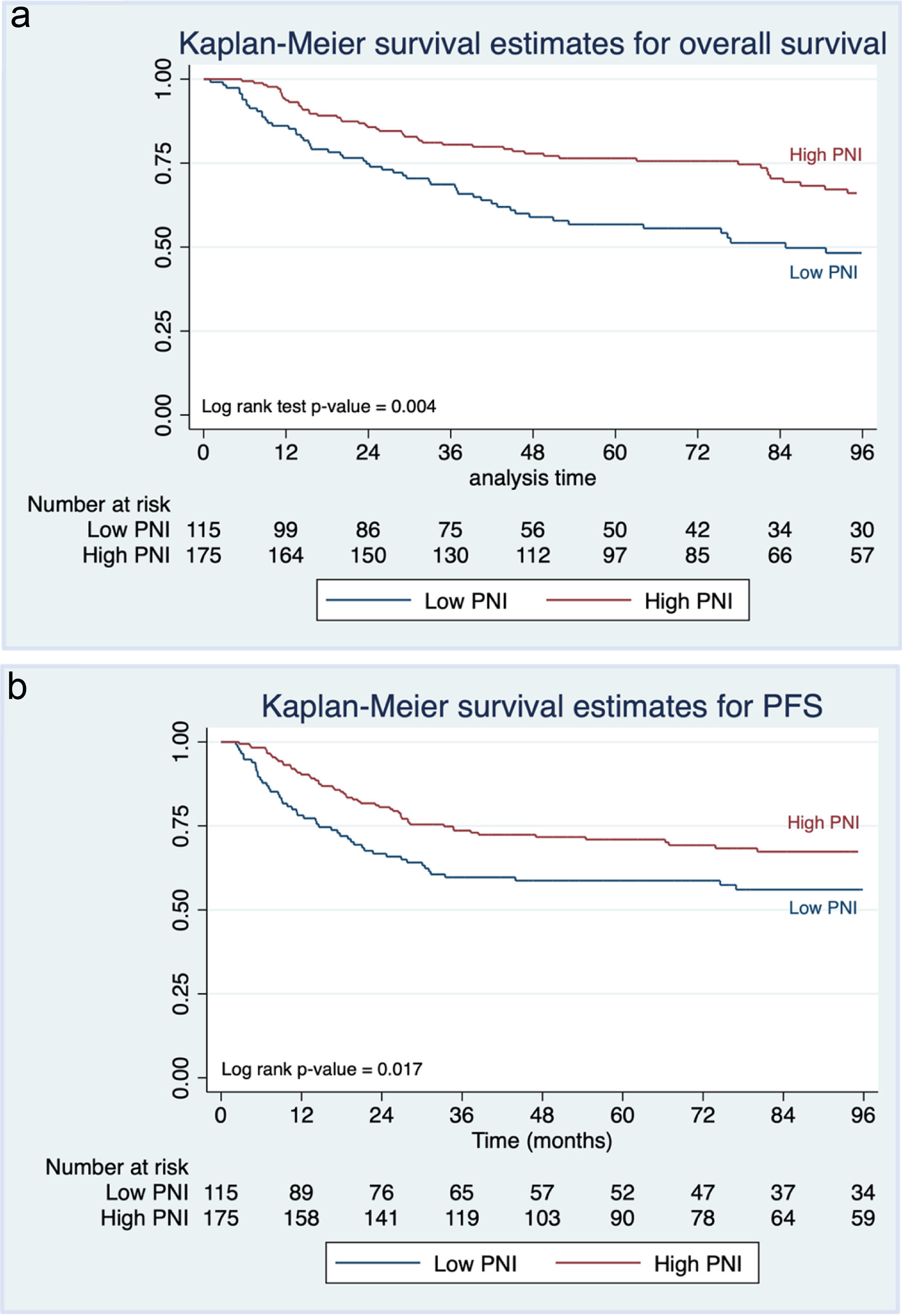 Click for large image | Figure 1. Kaplan-Meier curves of (a) overall survival and (b) progression-free survival of epithelial ovarian clear cell cancer patients stratified by preoperative prognostic nutritional index (PPNI). |
Table 3 displays 5-year survival rates in EOC-CC patients stratified by PPNI. The 5-year OS rates and 5-year PFS rates were poorer in the low PPNI group compared to the high PPNI group (5-year OS rate 56.8% vs. 76.4% and 5-year PFS rates 58.7% vs. 71.0%). Herein, we observed significant differences in BMI, CA125 level, albumin level, lymphocyte count, hemoglobin level, platelet count, cancer stage, optimal surgery, and ascites cytology between EOC-CC patients with low and high PPNI. These confounding factors could influence patient status, treatment modalities, and survival outcomes. We then adjusted these factors before summarizing our study’s conclusion. Surprisingly, the adjusted OS suggested a trend toward a more favorable outcome in the low PPNI group (Fig. 2), while adjusted PFS showed no difference.
 Click to view | Table 3. Five-Year Survival Rates of Patients With EOC-CC Stratified by PPNI |
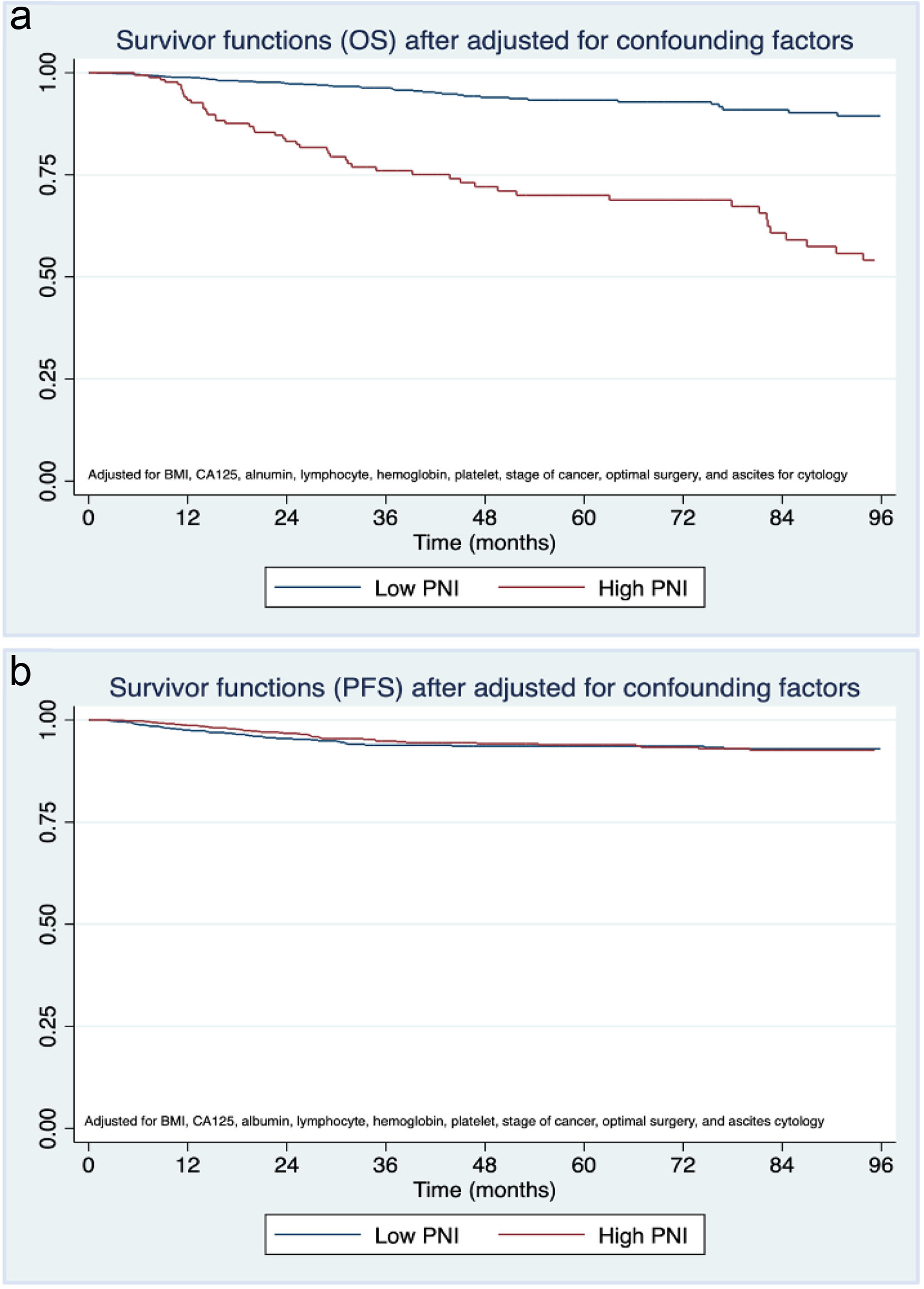 Click for large image | Figure 2. Kaplan-Meier curves of (a) overall survival and (b) progression-free survival of epithelial ovarian clear cell cancer patients stratified by preoperative prognostic nutritional index (PPNI) after adjusting for body mass index (BMI), cancer antigen 125 (CA125) level, albumin level, lymphocyte count, hemoglobin level, platelet count, stage of cancer, optimal surgery, and ascites cytology. |
A significant difference in albumin levels and lymphocyte counts was observed between low and high PPNI, as shown in Table 1. Accordingly, we conducted additional survival analysis stratified by albumin levels and lymphocyte counts. Kaplan-Meier curves (Supplementary Material 1, www.wjon.org) revealed significantly shorter OS and PFS in patients with low albumin levels (< 3.5 g/dL) compared to those with higher levels (≥ 3.5 g/dL) (log-rank P < 0.05). Similarly, Kaplan-Meier curves (Supplementary Material 2, www.wjon.org) showed significantly shorter OS and PFS in patients with low lymphocyte counts (< 30%) compared to those with higher lymphocyte counts (≥ 30%) (log-rank P < 0.05).
Table 4 presents univariate and multivariate analyses for survival outcomes in EOC-CC patients. Univariate analysis revealed that PPNI < 50, BMI ≤ 25 kg/m2, presence of underlying disease, serum CA125 ≥ 35 U/mL, serum albumin < 3.5 g/dL, lymphocyte count < 30%, hemoglobin level < 10 g/dL, platelet counts ≥ 350 × 103 cells/mm3, advanced FIGO stage, no optimal surgery, and positive cytology of ascites, were significant poor prognostic factors for PFS. After multivariate analyses, only the advanced FIGO stage independently predicted shorter PFS (adjusted HR: 7.30; 95% CI: 4.38 - 12.17). Regarding OS, we identified serum CA125, serum albumin, lymphocyte count, hemoglobin level, platelet count, FIGO stage, optimal surgery, ascites cytology, and the number of adjuvant chemotherapy cycles were significant poor prognostic factors for OS from univariate analysis. Subsequently, only serum CA125 ≥ 35 U/mL, advanced FIGO stage, and receiving chemotherapy ≤ 3 cycles remained independently associated with shorter OS after multivariate analyses. Nevertheless, PPNI did not significantly affect PFS or OS (95% CI include 1).
 Click to view | Table 4. Univariate and Multivariate Analyses for Survival Outcomes According to Individual Parameters |
| Discussion | ▴Top |
Clear cell cancer is relatively uncommon in EOC but more common in Asians, with a rising incidence [22]. Even though most EOC-CC patients are diagnosed early, patients in stage IC with positive peritoneal cytology or ascites have a worse prognosis. This finding occurs because the clear cell histology had a high recurrent rate, aggressive behavior, and resistance to platinum chemotherapy [23]. Therefore, identifying prognostic indicators is important for EOC-CC’s risk stratification and treatment planning.
Acknowledging the essential role of preoperative nutritional and immunologic status in cancer patient’s well-being, postoperative complications, and survival, we focused on the PPNI, derived from serum albumin and lymphocyte count in peripheral blood, which serves as a validated prognostic indicator in various cancers, predicting cancer-related mortality [11, 18, 24-26]. Nevertheless, its specific role in EOC-CC remains unclear, as previous studies encompassed all advanced EOC cases, which often involve malnutrition due to late diagnosis and extensive cancer progression. To address this gap, we conducted a retrospective cohort study to explore the association between PPNI and survival outcomes in EOC-CC patients.
The main finding of this study indicated a significant difference in PFS and OS between EOC-CC patients with low PPNI and high PPNI in univariate analysis, consistent with prior research (HR: 0.102; 95% CI: 0.008 - 0.602) [19]. However, in our multivariate analysis, PPNI did not independently predict OS and PFS. This could be due to variations in patient characteristics. Prior research focused solely on early-stage EOC-CC patients (stage I-II), with a PPNI cut-off level of 46.7, while our study included both early and advanced stages of EOC-CC patients with a slightly higher PPNI cut-off level of 50 [19]. The reason for this discrepancy in cut-off levels remains unclear and could be influenced by factors such as patent age, cancer stage, and ethnicity. Further investigations are needed to determine the optimal PPNI threshold in future studies.
The cut-off value of PPNI has been determined using various methods, including receiver operating characteristic (ROC) curves, median, mean, or multiple logistic regression analysis, to distinguish between normal and low PPNI statuses. Although their cut-off values varied across studies, they consistently fell within the range of 40 to 50 [18]. Similar to our study, we used the optimal cut-off value based on the mean value for our entire study population, which was 50. However, validation is necessary to confirm PPNI’s prognostic significance and optimal EOC-CC cut-off value. Thus, establishing a normal PPNI range requires epidemiological research to categorize patients as high or low PPNI.
Regarding clinical characteristics in our study, we found that patients had a mean age at the time of diagnosis of 53 years, consistent with previous research reporting a median age ranging from 49 to 54 years, which is younger than those with serous EOC [27, 28]. Furthermore, preoperative serum CA125 was elevated in 75.2% of EOC-CC patients, aligning with previous finding [8]. Elevated serum CA125 may be associated with the presence of endometriosis lesions, which increases the risk of EOC-CC [29]. Although we plan to record the details of endometriosis-associated OC in this study, we could not include endometriosis-related data due to the retrospective nature and incomplete medical records. Hence, investigating endometriosis-associated EOC-OC remains important for future epidemiologic studies on EOC-CC patient prognosis.
The present study found a higher proportion of low PPNI in advanced-stage EOC-CC (36.5% vs. 14.9%, P < 0.001), consistent with Lee et al’s findings, which concluded that a decreased PPNI was associated with shorter survival outcomes especially in stage III OC [27]. This may be explained by the increased production of cytokines (e.g., interleukin-6 and tumor necrosis factor) from the advanced tumors, which inhibit lymphopoiesis and induce inflammatory activity, resulting in hypoalbuminemia [30, 31].
The strengths of this study included the new concept of identification of prognostic indicators in patients with EOC-CC through a simple method and low-cost blood examination, which is routinely conducted before surgery without incurring additional expenses. The study benefits from a relatively long follow-up period, with an average duration of 63 months for most patients. This duration is sufficient to monitor recurrence or mortality events in EOC-CC patients. Additionally, the study exclusively included patients treated at the same institution to ensure a homogenous group and minimize the variation in operative techniques and treatment protocols that could influence patient outcomes.
Nevertheless, the present study has several limitations that warrant consideration for future investigations in this area. Firstly, our study is constrained by its retrospective nature, which is susceptible to recall and measurement biases and may not fully account for all confounding factors. Secondly, although this study was conducted within a single institution with consistent operative techniques and treatment protocols, generalizability to other populations may be limited. Finally, despite adjusting for significant prognostic factors, including surgical stages, and our databases enabling assessment of cancer stage at the initial diagnosis, we did not capture the full extent of disease severity and actual residual disease. Therefore, some residual confounding factors (e.g., endometriosis-associated lesions, pelvic adhesion, and residual disease) could have been present and may have influenced our participants’ recurrence and mortality rates.
Conclusion
Our findings indicate that EOC-CC patients with low PPNI are at a higher risk of worse OS and PFS. Thus, PPNI may prove valuable for predicting outcomes and optimizing treatment strategies for EOC-CC patients. However, future prospective multicenter clinical research with larger sample sizes is needed to establish its clinical utility conclusively.
| Supplementary Material | ▴Top |
Suppl 1. Kaplan-Meier curves of (a) overall survival and (b) progression-free survival of epithelial ovarian clear cell cancer patients stratified by albumin levels.
Suppl 2. Kaplan-Meier curves of (a) overall survival and (b) progression-free survival of epithelial ovarian clear cell cancer patients stratified by lymphocyte counts.
Acknowledgments
The authors thank all participants, the research team, and gynecologic oncologists of the Division of Gynecologic Oncology, Department of Obstetrics and Gynecology, Rajavithi Hospital, for their valuable contributions to this study.
Financial Disclosure
The Rajavithi research management fund supported the present study.
Conflict of Interest
The authors declared that there was no conflict of interest.
Informed Consent
This study was approved by the Institutional Review Board (IRB) of Rajavithi Hospital (No. 63225). Informed consent was waived due to the retrospective nature of the study.
Author Contributions
Nisa Prueksaritanond: conceptualization; formal analysis; investigation; supervision; writing-original draft; writing-review and editing. Kittisak Petchsila: conceptualization; investigation; writing-original draft. Putsarat Insin: formal analysis; writing-original draft; writing-review and editing. All authors have reviewed and provided consent for publication.
Data Availability
The data supporting this study’s findings are available from the corresponding author upon reasonable request.
| References | ▴Top |
- Prat J, D'Angelo E, Espinosa I. Ovarian carcinomas: at least five different diseases with distinct histological features and molecular genetics. Hum Pathol. 2018;80:11-27.
doi pubmed - Gadducci A, Multinu F, Cosio S, Carinelli S, Ghioni M, Aletti GD. Clear cell carcinoma of the ovary: Epidemiology, pathological and biological features, treatment options and clinical outcomes. Gynecol Oncol. 2021;162(3):741-750.
doi pubmed - Anglesio MS, Carey MS, Kobel M, Mackay H, Huntsman DG, Vancouver Ovarian Clear Cell Symposium S. Clear cell carcinoma of the ovary: a report from the first Ovarian Clear Cell Symposium, June 24th, 2010. Gynecol Oncol. 2011;121(2):407-415.
doi pubmed - Iida Y, Okamoto A, Hollis RL, Gourley C, Herrington CS. Clear cell carcinoma of the ovary: a clinical and molecular perspective. Int J Gynecol Cancer. 2021;31(4):605-616.
doi pubmed - Chan JK, Teoh D, Hu JM, Shin JY, Osann K, Kapp DS. Do clear cell ovarian carcinomas have poorer prognosis compared to other epithelial cell types? A study of 1411 clear cell ovarian cancers. Gynecol Oncol. 2008;109(3):370-376.
doi pubmed - Lee YY, Kim TJ, Kim MJ, Kim HJ, Song T, Kim MK, Choi CH, et al. Prognosis of ovarian clear cell carcinoma compared to other histological subtypes: a meta-analysis. Gynecol Oncol. 2011;122(3):541-547.
doi pubmed - Itamochi H, Kigawa J, Terakawa N. Mechanisms of chemoresistance and poor prognosis in ovarian clear cell carcinoma. Cancer Sci. 2008;99(4):653-658.
doi pubmed - Tian C, Markman M, Zaino R, Ozols RF, McGuire WP, Muggia FM, Rose PG, et al. CA-125 change after chemotherapy in prediction of treatment outcome among advanced mucinous and clear cell epithelial ovarian cancers: a Gynecologic Oncology Group study. Cancer. 2009;115(7):1395-1403.
doi pubmed - Garth AK, Newsome CM, Simmance N, Crowe TC. Nutritional status, nutrition practices and post-operative complications in patients with gastrointestinal cancer. J Hum Nutr Diet. 2010;23(4):393-401.
doi pubmed - Savant SS, Sriramkumar S, O'Hagan HM. The role of inflammation and inflammatory mediators in the development, progression, metastasis, and chemoresistance of epithelial ovarian cancer. Cancers (Basel). 2018;10(8):251.
doi pubmed - Onodera T, Goseki N, Kosaki G. [Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients]. Nihon Geka Gakkai Zasshi. 1984;85(9):1001-1005.
pubmed - Ayhan A, Gunakan E, Alyazici I, Haberal N, Altundag O, Dursun P. The preoperative albumin level is an independent prognostic factor for optimally debulked epithelial ovarian cancer. Arch Gynecol Obstet. 2017;296(5):989-995.
doi pubmed - Lee YJ, Chung YS, Lee JY, Nam EJ, Kim SW, Kim S, Kim YT. Pretreatment lymphocytopenia is an adverse prognostic biomarker in advanced-stage ovarian cancer. Cancer Med. 2019;8(2):564-571.
doi pubmed - Jiang N, Deng JY, Ding XW, Ke B, Liu N, Zhang RP, Liang H. Prognostic nutritional index predicts postoperative complications and long-term outcomes of gastric cancer. World J Gastroenterol. 2014;20(30):10537-10544.
doi pubmed - Ikeya T, Shibutani M, Maeda K, Sugano K, Nagahara H, Ohtani H, Hirakawa K. Maintenance of the nutritional prognostic index predicts survival in patients with unresectable metastatic colorectal cancer. J Cancer Res Clin Oncol. 2015;141(2):307-313.
doi pubmed - Hong S, Zhou T, Fang W, Xue C, Hu Z, Qin T, Tang Y, et al. The prognostic nutritional index (PNI) predicts overall survival of small-cell lung cancer patients. Tumour Biol. 2015;36(5):3389-3397.
doi pubmed - Haraga J, Nakamura K, Omichi C, Nishida T, Haruma T, Kusumoto T, Seki N, et al. Pretreatment prognostic nutritional index is a significant predictor of prognosis in patients with cervical cancer treated with concurrent chemoradiotherapy. Mol Clin Oncol. 2016;5(5):567-574.
doi pubmed - Dai Y, Liu M, Lei L, Lu S. Prognostic significance of preoperative prognostic nutritional index in ovarian cancer: A systematic review and meta-analysis. Medicine (Baltimore). 2020;99(38):e21840.
doi pubmed - Yoshikawa N, Yoshida K, Tamauchi S, Ikeda Y, Nishino K, Niimi K, Suzuki S, et al. The preoperative prognostic nutritional index for the prediction of outcomes in patients with early-stage ovarian clear cell carcinoma. Sci Rep. 2020;10(1):7135.
doi pubmed - Prat J, Oncology FCoG. Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet. 2014;124(1):1-5.
doi pubmed - Bernard. Fundamentals of biostatistics, 5th ed. Thomson Learning, Duxbery. 2000. p. 384-385.
- Sung PL, Chang YH, Chao KC, Chuang CM, Task Force on Systematic R, Meta-analysis of Ovarian C. Global distribution pattern of histological subtypes of epithelial ovarian cancer: a database analysis and systematic review. Gynecol Oncol. 2014;133(2):147-154.
doi pubmed - Hogen L, Vicus D, Ferguson SE, Gien LT, Nofech-Mozes S, Lennox GK, Bernardini MQ. Patterns of recurrence and impact on survival in patients with clear cell ovarian carcinoma. Int J Gynecol Cancer. 2019;29(7):1164-1169.
doi pubmed - Gupta D, Lis CG. Pretreatment serum albumin as a predictor of cancer survival: a systematic review of the epidemiological literature. Nutr J. 2010;9:69.
doi pubmed - Miao Y, Li S, Yan Q, Li B, Feng Y. Prognostic significance of preoperative prognostic nutritional index in epithelial ovarian cancer patients treated with platinum-based chemotherapy. Oncol Res Treat. 2016;39(11):712-719.
doi pubmed - Zhang W, Ye B, Liang W, Ren Y. Preoperative prognostic nutritional index is a powerful predictor of prognosis in patients with stage III ovarian cancer. Sci Rep. 2017;7(1):9548.
doi pubmed - Lee HY, Hong JH, Byun JH, Kim HJ, Baek SK, Kim JY, Kim KH, et al. Clinical Characteristics of clear cell ovarian cancer: a retrospective multicenter experience of 308 patients in South Korea. Cancer Res Treat. 2020;52(1):277-283.
doi pubmed - Timmers PJ, Zwinderman AH, Teodorovic I, Vergote I, Trimbos JB. Clear cell carcinoma compared to serous carcinoma in early ovarian cancer: same prognosis in a large randomized trial. Int J Gynecol Cancer. 2009;19(1):88-93.
doi pubmed - Pearce CL, Templeman C, Rossing MA, Lee A, Near AM, Webb PM, Nagle CM, et al. Association between endometriosis and risk of histological subtypes of ovarian cancer: a pooled analysis of case-control studies. Lancet Oncol. 2012;13(4):385-394.
doi pubmed - Shioya M, Yoshida T, Kasai K, Furuya R, Kato A, Mori N, Matsumoto Y, et al. Inflammatory factors for hypoalbuminemia in Japanese peritoneal dialysis patients. Nephrology (Carlton). 2013;18(8):539-544.
doi pubmed - Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6.Sle1.Yaa animals. Blood. 2009;113(19):4534-4540.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.