| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 1, February 2023, pages 75-83
High Body Mass Index Was Associated With Human Epidermal Growth Factor Receptor 2-Positivity, Histological Grade and Disease Progression Differently by Age
Di Zhaoa, f, Xiaoyan Wanga, f, Narasimha M. Beerakab, c, d, f, Runze Zhoub, e, Haohao Zhanga, Yanxia Liua, Yinghui Zhanga, Ying Zhanga, Guijun Qina, Junqi Liub, e, g
aEndocrinology Department, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450000, China
bDepartment of Radiation Oncology, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450000, China
cDepartment of Human Anatomy, I.M. Sechenov First Moscow State Medical University (Sechenov University), Moscow 119991, Russia
dDepartment of Pharmaceutical Chemistry, JSS Academy of Higher Education and Research (JSS AHER), JSS College of Pharmacy, Mysuru, Karnataka, India
eCancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450000, China
fThese authors contributed equally to this article.
gCorresponding Author: Junqi Liu, Cancer Center, The First Affiliated Hospital of Zhengzhou University, Zhengzhou, Henan Province 450000, China
Manuscript submitted November 11, 2022, accepted February 6, 2023, published online February 26, 2023
Short title: Prognosis of Breast Cancer Patients and BMI
doi: https://doi.org/10.14740/wjon1543
| Abstract | ▴Top |
Background: Breast cancer is the most commonly occurring cancer among women. The relationship between the obesity paradox and breast cancer is still unclear. The goal of this study is to elucidate the association between high body mass index (BMI) and pathological findings by age.
Methods: We collected BMI information pertinent to breast cancer patients from the Gene Expression Omnibus (GEO) database. We use a BMI of 25 as a boundary, and those greater than 25 are defined as high BMI. Besides, we segregated the patients based on age into two age groups: < 55 years, and > 55 years. In this study, R × C Chi-square for trend and binary logistic regression was used to estimate the odds ratios (ORs) and corresponding 95% confidence intervals (CIs).
Results: Higher BMI was associated with less breast cancer incidence in females younger than 55 years of age (OR = 0.313, CI: 0.240 - 0.407). High BMI was associated with human epidermal growth factor receptor 2 (HER2) positivity in breast cancer patients of less than 55 years (P < 0.001), but not in the older patients. High BMI was associated with histological grade lower than 2 in the breast cancer patients older than 55 years, but not in younger patients (OR = 0.288, CI: 0.152 - 0.544). Besides, high BMI was associated with worse progression-free survival in younger breast cancer patients, but not in older patients (P < 0.05).
Conclusions: Our results described a significant relationship between breast cancer incidence and BMI at different ages and benefit breast cancer patients to implement strategies to control their BMI for reducing the recurrence and distant recurrence.
Keywords: Body mass index; HER2; Breast cancer; Prognosis; Age
| Introduction | ▴Top |
Breast cancer is the most commonly occurring cancer in females worldwide [1-8]. According to GLOBOCAN reports, breast cancer is the leading cause of cancer deaths in women from 20 to 59 years of age [9]. Currently, the risk factors for breast cancer include smoking, delayed childbirth, shorter breastfeeding, alcohol consumption, and environmental and genetic factors [10, 11].
Overweight and obesity is a chronic metabolic disease caused by multiple factors such as heredity and environment [12, 13]. In addition, studies have shown that overweight and obesity are related to the occurrence and development of many diseases, such as diabetes, fatty liver, cardiovascular disease, and various cancers [14-17]. Obesity is one of the significant factors for poor prognosis in breast cancer patients. Overweight may be conducive to a higher tumor stage and tumor grade accompanied by distant recurrence, and metastases of luminal molecular subtypes in breast cancer patients. Now-a-days, several reports describe the relationship between obesity and breast cancer incidence [18].
According to the National Institutes of Health (NIH) and World Health Organization (WHO), the body mass index (BMI) is a statistical validity index to evaluate body fat in men and women of any age [19, 20]. However, obesity has no significant influence on the specific overall survival in breast cancer conditions mainly among patients with human epidermal growth factor receptor 2 (HER2)-positive or triple-negative breast cancers (TNBCs). But obesity could influence the overall survival of hormone-specific breast cancer partly by harnessing poor prognosis [21]. A plethora of reports delineated that BMI status can contribute to clinical outcomes in breast cancer patients. In a recent randomized clinical trial of 5,683 patient cohorts, the patients with BMI > 35 were typically associated with a higher risk of recurrence than the patients with BMI < 25 [1-8]. Yet significant research is required to explore the relationship between obesity and clinical outcomes in breast cancer. The effect of BMI during obesity on clinical outcomes in breast cancer patients with recurrence rate was reported through retrospective analysis of SUCCESS trial [22]. Women with obesity exhibited a significant rise in acquiring stage III/IV disease and grade 3/4 tumors in breast cancer conditions [21]. However, the relationship between obesity and breast cancer incidence is still unclear. This study aims to ascertain “the impact of high BMI” reported at different ages on the incidence of breast cancer by deciphering the BMI information of breast cancer patients obtained from the Gene Expression Omnibus (GEO) database. This article provides a prognostic strategy to decipher the impact of “high BMI at different ages” pertinent to the incidence of breast cancer. In addition, the difference between breast cancer patients with different BMI at different ages was delineated.
| Materials and Methods | ▴Top |
Data collection
We entered the keyword search, “BMI and cancer” in the NCBI-GEO database [23] and obtained 7,449 search records; among them, 48 GSE datasets contained information pertinent to patient’s BMI and cancers such as breast cancer, prostate cancer, esophagus cancer, and ovarian cancer. Due to the large abundance of breast cancer data, the GSE datasets containing other tumor information were deleted, and GSE datasets with a sample size of less than 100 were also deleted. GSEs that contain clinical information pertinent to breast cancer patients include GSE24185, GSE54470, GSE70905, GSE70947, GSE78958, GSE93601, GSE115577, whereas the information pertinent to the normal breast tissues includes GSE101961, GSE102088, GSE15289, GSE43973, GSE88781, GSE88782, GSE88783, and GSE88785. R statistical computing software was used to download their clinical information in the GSE dataset. Grouping was performed according to the BMI classification guidelines given by WHO: BMI < 18.5 are considered underweight, whereas BMI of 18.5 - 24.9 are normal, 25.0 - 29.0 is overweight, and ≥ 30.0 are obese, respectively. The overall proportion of obese people is minimal; therefore, we combined the overweight and obese people together and collectively considered to envisage the influence of high BMI on the incidence of breast cancer. Fewer individuals were underweight among the participants. Hence, the data of these people were not considered for further analysis. Due to the incomplete information of a few samples in GSE, we need to exclude the “not available (NA)” data in the statistics.
Statistical analyses
Statistical analysis was performed using the SPSS statistical package (version IBM SPSS Statistics 21), GraphPad Prism 8.0.1 with P < 0.05 and P < 0.001 indicating statistical significance. R × C Chi-square was performed for ascertaining the trends while binary logistic regression was used to estimate the odds ratio (OR) and corresponding 95% confidence intervals (CIs).
Statement of ethics
An ethics statement was not required for this study type, and no human or animal subjects or materials were used. This study does not involve any human participant and does not require informed content. The whole study includes datasets acquired from the GEO, a public database, and was executed with complete institutional approval from The First Affiliated Hospital of Zhengzhou University.
| Results | ▴Top |
GSE samples containing BMI information (4,132) were divided into two groups: 2,507 patients with breast cancer and 1,625 patients in the control group to determine the frequency of breast cancer and its correlation with BMI (Table 1). Among the 4,132 samples with BMI information, Chi-square test reported that the incidence of breast cancer is different among different age groups (P < 0.001) (Table 2). The baseline characteristics of all the patients in this study were given in the Table. 1.
 Click to view | Table 1. Baseline Characteristics of Patient Demographics by BMI |
 Click to view | Table 2. Chi-Square Test Results Between Breast Cancer Patient Demographics by BMI and Age and Individuals Without Breast Cancer |
A significant association was observed between HER2 positivity and BMI status among the age group of breast cancer patients less than 55 years (P < 0.001) (Table 3). Among the age group of breast cancer patients older than 55 years, Chi-square results described the significant difference in the tumor grade between different BMI groups (P < 0.001) (Table 3).
 Click to view | Table 3. Chi-Square Test for BMI Classification in the Patients With Breast Tumors at Different Ages |
The patient’s age group less than 55 years with high BMI are more likely to be HER2 positive (OR = 4.795, 95% CI = 2.517 - 9.136, P < 0.001) when compared to the breast cancer patients with normal weight (Fig. 1a). Furthermore, breast cancer patients older than 55 years were accompanied by high BMI, and they are more likely to have a tumor grade greater than 2 (OR = 0.288, 95% CI = 0.152 - 0.544, P < 0.001) (Fig. 1b) when compared to the breast cancer patients with normal weight (Table 4).
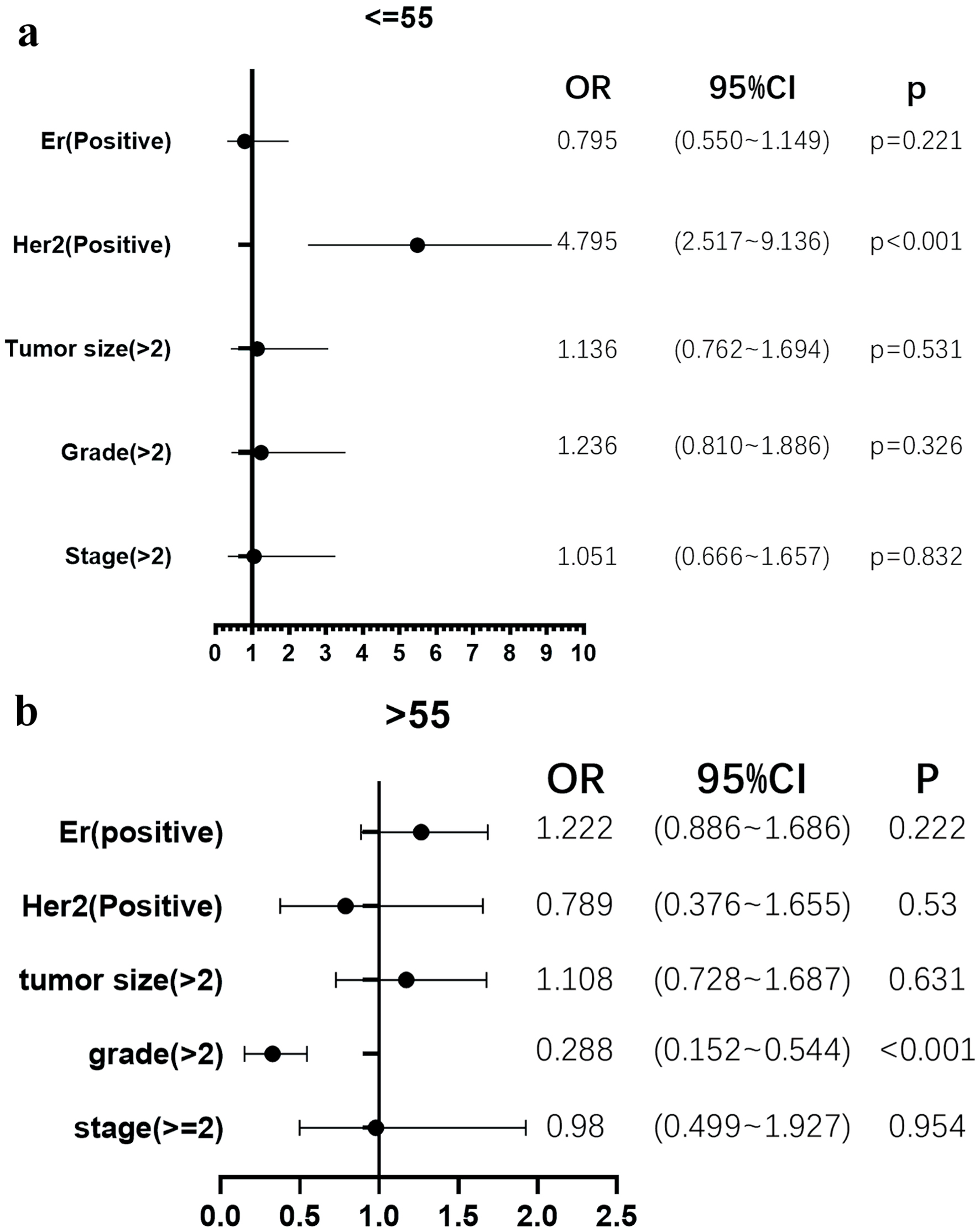 Click for large image | Figure 1. High body mass index (BMI) was associated with HER2 positivity in younger, but not in older patients, and was associated with lower histological grade in older, but not in younger patients. (a) The patient’s age group less than 55 years with high BMI are more likely to be HER2 positive (odds ratio (OR) = 4.795, 95% confidence interval (CI) = 2.517 - 9.136, P < 0.001) when compared to the breast cancer patients with normal weight. (b) Breast cancer patients older than 55 years were accompanied by high BMI, and they are more likely to have a tumor grade greater than 2 (OR = 0.288, 95% CI = 0.152 - 0.544, P < 0.001). |
 Click to view | Table 4. The Logistics Regression Analysis of BMI Classification of Tumor Patients at Different Ages |
In this study, both recurrence and distant recurrence were considered as “disease progression”. High BMI was associated with worse progression-free survival (PFS) in younger, but not in older patients. Figure 2a depicts that the breast cancer patients with high BMI are more likely to have “recurrence and distant recurrence” in a relatively short period (P = 0.0023), when compared to normal BMI patients mainly among breast cancer patients (< 55 years). However, this difference was not observed in breast cancer patients of age group older than 55 years (P = 0.4597) (Fig. 2b). Additional information related to results between breast cancer patients of different ages and people without breast cancer can be found here (Supplementary Material 1, www.wjon.org), as well the BMI classification of breast cancer patients at different ages (Supplementary Material 2, www.wjon.org). Furthermore, PFS periods of different ages by BMI categories are provided (Supplementary Material 3, www.wjon.org). Results between breast cancer patients of different ages and people without breast cancer are given here (Supplementary Material 4, www.wjon.org), whereas the results pertinent to BMI classification of breast cancer patients at different ages are provided (Supplementary Material 5, www.wjon.org). We also provided PFS periods of different ages by BMI categories (Supplementary Material 6, www.wjon.org).
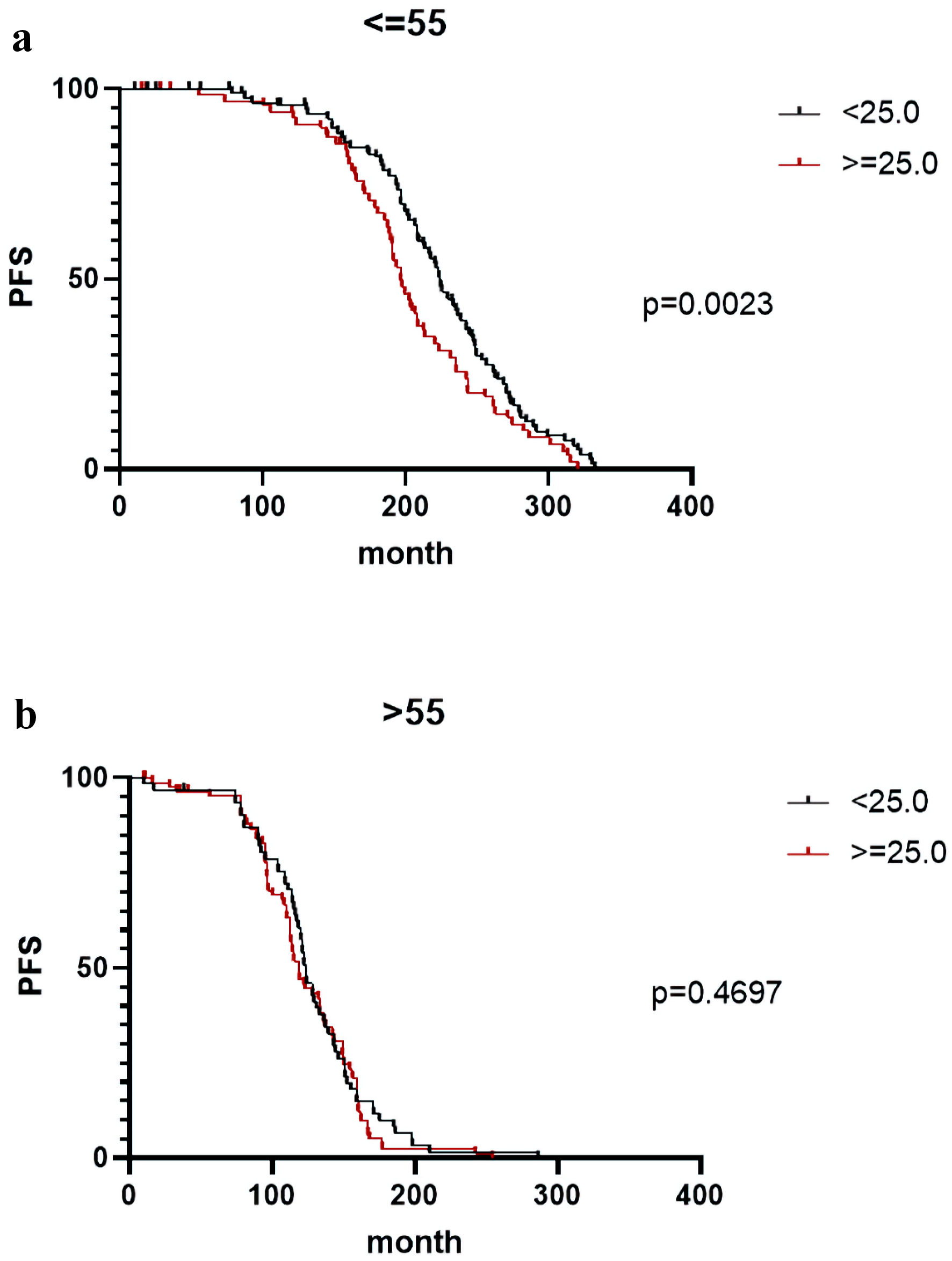 Click for large image | Figure 2. (a, b) High body mass index (BMI) was associated with worse progression-free survival (PFS) in younger, but not in older patients. Breast cancer patients with high BMI are more likely to have “recurrence and distant recurrence” in a relatively short span of time (P = 0.0023) when compared to the normal BMI patients mainly among the breast cancer patients younger than 55 years (a); however, this difference was not observed in breast cancer patients older than 55 years (P = 0.4597) (b). |
| Discussion | ▴Top |
BMI and breast cancer survival relationship can be deciphered depending on the obesity and prognosis-related tumor characteristics. Biological pathways significantly involved in the adiposity include the local and systemic release of “inflammatory markers, insulin growth factors, steroid receptors, adipokines”, which can affect several signaling cascades [8, 24] in breast cancer cells by modulating the JAK/STAT3 signaling, insulin/insulin-like growth factor (IGF) signaling, and estrogen signaling. Subsequently, these alterations can promote metabolic reprogramming, cancer cell proliferation, angiogenesis, and metastasis directly or indirectly in the tumor microenvironment [23, 25-30]. Normally, women with obesity and breast cancer exhibit typically a higher cancer cell proliferation rate [28, 31]. Altered BMI could promote adverse tumor characteristics and suggest the possibility of delayed initial cancer detection as an alternate cause for the poor prognosis in obese women with breast cancer [28, 32, 33]. Failure in the initial detection could lead to the delayed identification of metastasis, and recurrence, and affect overall survival of obese women with breast cancer. The current study is based on the GEO database to analyze the relationship between BMI and breast cancer at different ages. Our study reported that the difference in the BMI status between breast cancer patients and non-breast cancer individuals is not statistically significant. In addition, the logistic regression analysis reported that women with less than 55 years of age are accompanied by a higher risk of getting breast cancer than women older than 55 years. High BMI was associated with HER2 positivity in younger, but not in older patients, and was associated with lower histological grade in older, but not in younger patients. Besides, the breast cancer patients with relatively higher BMI exhibited an increased risk of pathological grade greater than 2 when compared to the patients with normal BMI (> 55 years old). Our results showed that the patients with high BMI are more likely to possess recurrence or distant metastasis in a relatively short period of time.
Overweight and obesity can be referred to as significant predictors for higher risk of 5-year breast cancer relapse and mortality [13, 33]. Another study by Jiralerspong et al (2013) [34] described the hazard ratios for the risk of recurrence and mortality in breast cancer patients with obesity (relative to the normal weight) as 1.18 (95% CI: 1.02 - 1.36) and 1.23 (95% CI: 1.00 - 1.52), respectively. According to this report, the overweight did not influence the recurrence and suggested that obesity was not a predictor for mortality in breast cancer patients [34]. In African TNBC patients, the mortality and recurrence of breast cancer patients were related to the overweight [34]. The risk of acquiring distant metastasis has a significant correlation with obesity in Danish breast cancer patients and suggested that both overweight, and obesity in women mitigated the overall survival in breast cancer patients when compared to the patients with normal weight [34]. This may be related to differences in steroid receptor expression, and lncRNAs at the molecular level in young breast cancer patients, which requires extensive molecular studies [35]. However, some studies stated that aging is a risk factor for breast cancer [36-38]. Therefore, the relationship between age and breast cancer incidence may be more complicated and require substantial studies to elucidate the underlying mechanisms.
BMI status in premenopausal women is negatively correlated with the risk of acquiring breast cancer [16, 39-42]. This may be related to the higher incidence of irregular menstruation and anovulatory cycles in women with high BMI [43]. In addition, studies have shown that women with high BMI are associated with low amounts of estradiol and progesterone than the women with normal BMI [44, 45]. Another study by Widschwendter et al concluded that chronic obesity with BMI greater than 40 is reported with worse clinical outcomes in high-risk early TNBCs [22]. Furthermore, it has been suggested that obese and postmenopausal women could have an increased risk of breast cancer [16, 40, 46-48]. The risk of acquiring stage III/IV and grade 3/4 tumors is substantially higher in obese women; luminal A and luminal B molecular subtypes in obese patients are likely associated with low overall survival than the patients with normal weight [21]. Pajares et al observed poorer clinical outcomes in breast cancer patients with BMI greater than 35 when compared to the patients with BMI less than 25 and subsequently concluded the “effect of BMI” was attributed to being same breast cancer subtypes such as estrogen receptor (ER)/ progesterone receptor (PR) (+), HER (-)/HER (+), TNBCs [49]. In addition, the higher mortality rate in obese women with breast cancer is prominently due to the ER+/luminal molecular subtypes [22, 34, 50-57]. However, our results reported that the association between obesity and postmenopausal breast cancer is not obvious, which may be due to our limited statistical data.
The three adjuvant clinical trials pertinent to “Eastern Cooperative Oncology Group” were executed for anthracycline-based chemotherapy; and the results of this study reported poor clinical outcomes in breast cancer patients with BMI > 30 when compared to nonobese patients with hormone receptor (HR)-positive disease [22]. A previous study by Blair et al concluded that BMI status exhibited significant relation with all-cause mortality in breast cancer patients [21]. Another study [57] deciphered the effect of BMI on disease-free survival and overall survival of breast cancer patients who received neoadjuvant chemotherapy [57]; and according to this study, the patients with BMI of 30 - 40 and > 40 exhibited relatively minimal disease-free survival and overall survival when compared to the patients with normal weight [57]. In another study, it is evident that the BMI has no significant effect on the overall survival in luminal subtype (ER/PR (+), and HER (-)), and TNBCs but has a profound effect in HER (+) tumors [22]. Our finding showed that patients with high BMI are more likely to be HER2 positive, among the age group of breast cancer patients less than 55 years. High BMI was associated with worse PFS in younger, but not in older patients. However, substantial studies are required to understand the risk factors associated with HER2 subtypes. Furthermore, we concluded that high BMI in breast cancer patients exhibited a pathological grade greater than 2 among patients older than 55 years of age. Therefore, the tumor progression in breast cancer patients with high BMI is relatively faster than those patients with normal BMI. Certain studies already proved that obesity can lead to distant recurrence of breast cancer [58].
Limitations
The quality of the original data cannot be completely controlled, because of the samples from different GSE databases, which inevitably produces deviations. These clinically significant findings support the need for clinical trials to evaluate the effects of obesity prevention and treatment on breast cancer risk. There is no available information in the GEO database pertinent to the breast cancer subtype or treatment data to demonstrate substantial prognostic information. We gathered the information for the generalizable prognostic information based on potentially more determinative variables such as age, and BMI that would benefit effective clinical management.
| Supplementary Material | ▴Top |
Suppl 1. Chi-square test results between breast cancer patients of different ages and people without breast cancer.
Suppl 2. Chi-square test of BMI classification of breast cancer patients at different ages.
Suppl 3. PFS period of different ages by BMI categories.
Suppl 4. Chi-square test results between breast cancer patients of different ages and people without breast cancer.
Suppl 5. Chi-square test of BMI classification of breast cancer patients at different ages.
Suppl 6. The PFS period of different ages by BMI categories.
Acknowledgments
The authors convey sincere thanks to the administrative and technical staff of the Department of Radiation Oncology and Endocrinology Department of The First Affiliated Hospital of Zhengzhou University. Our sincere thanks to Professor Ruitai Fan, MD, Ph.D., director & chairman of Cancer Center, The First Affiliated Hospital of Zhengzhou University for his support during this work.
Financial Disclosure
This study was supported by National Natural Science Foundation of China (No. 81700729), and Henan Province Medical Science and Technology Research Project (No. LHGJ20190249).
Conflict of Interest
The authors declare no conflict of interest.
Informed Consent
This study does not involve any human participants and does not require informed content. The whole study included datasets acquired from the GEO, a public database and was executed with complete institutional approval from the First Affiliated Hospital of Zhengzhou University.
Author Contributions
Di Zhao (DZ), Narasimha M. Beeraka (NMB), Xiaoyan Wang (XW), Runze Zhou (RZ), Haohao Zhang (HZ), Yanxia Liu (YL), Yinghui Zhang (YGZ), Ying Zhang (YZ), Junqi Liu (JL), and Guijun Qin (GQ) conceptualized and designed the study. NMB, DZ, XW, RZ, HZ, YL, YZ, JL, and GQ performed the literature analysis, and wrote the original manuscript draft. NMB, JL, GQ revised, edited, and extended the final draft. All authors have reviewed and approved the manuscript before submission.
Data Availability
This study was executed by acquiring the data from GEO, a public database. Readers can access the datasets as we described in the Methods section under data collection subheading.
Abbreviations
BMI: body mass index; GEO: Gene Expression Omnibus; CI: confidence interval; WHO: World Health Organization; OR: odds ratio; ER: estrogen receptor; HER2: human epidermal growth factor receptor 2
| References | ▴Top |
- Sancho-Garnier H, Colonna M. [Breast cancer epidemiology]. Presse Med. 2019;48(10):1076-1084.
doi pubmed - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi pubmed - Reeves GK, Beral V, Green J, Gathani T, Bull D, Million Women Study C. Hormonal therapy for menopause and breast-cancer risk by histological type: a cohort study and meta-analysis. Lancet Oncol. 2006;7(11):910-918.
doi pubmed - Chen K, N MB, Zhang J, Reshetov IV, Nikolenko VN, Sinelnikov MY, Mikhaleva LM. Efficacy of da Vinci robot-assisted lymph node surgery than conventional axillary lymph node dissection in breast cancer - A comparative study. Int J Med Robot. 2021;17(6):e2307.
doi - Chen K, Beeraka NM, Li J, Lu P. Anterior abdominal wall defect closed by the anterior sheath of the upper rectus abdominis muscle in a patient with prior "TRAM Flap Breast Reconstruction". Indian Journal of Surgery. 2022;84:789-791.
doi - Chubarev VN, Beeraka NM, Sinelnikov MY, Bulygin KV, Nikolenko VN, Mihaylenko E, Tarasov VV, et al. Health science community will miss this bright and uniting star: in: memory of professor Gjumrakch Aliev, M.D, Ph.D. Cancers (Basel). 2021;13(8):1965.
doi pubmed - Beeraka NM, Bovilla VR, Doreswamy SH, Puttalingaiah S, Srinivasan A, Madhunapantula SV. The taming of Nuclear Factor Erythroid-2-Related Factor-2 (Nrf2) Deglycation by Fructosamine-3-Kinase (FN3K)-Inhibitors - A novel strategy to combat cancers. Cancers (Basel). 2021;13(2):281.
doi pubmed - Reddy BD, Beeraka NM, Chitturi CMK, Madhunapantula SV. An Overview of Targeting Legumain for Inhibiting Cancers. Curr Pharm Des. 2021;27(31):3337-3348.
doi pubmed - Gansler T, Ganz PA, Grant M, Greene FL, Johnstone P, Mahoney M, Newman LA, et al. Sixty years of CA: a cancer journal for clinicians. CA Cancer J Clin. 2010;60(6):345-350.
doi pubmed - Jones ME, Schoemaker MJ, Wright LB, Ashworth A, Swerdlow AJ. Smoking and risk of breast cancer in the Generations Study cohort. Breast Cancer Res. 2017;19(1):118.
doi pubmed - Rojas K, Stuckey A. Breast cancer epidemiology and risk factors. Clin Obstet Gynecol. 2016;59(4):651-672.
doi pubmed - Hruby A, Hu FB. The epidemiology of obesity: a big picture. Pharmacoeconomics. 2015;33(7):673-689.
doi pubmed - Chen K, Zhang J, Beeraka NM, Tang C, Babayeva YV, Sinelnikov MY, Zhang X, et al. Advances in the prevention and treatment of obesity-driven effects in breast cancers. Front Oncol. 2022;12:820968.
doi pubmed - Panuganti KK, Nguyen M, Kshirsagar RK. Obesity. In: StatPearls. Treasure Island (FL). 2022.
- Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K, International Agency for Research on Cancer Handbook Working Group. Body fatness and cancer—viewpoint of the IARC Working Group. N Engl J Med. 2016;375(8):794-798.
doi pubmed - Bhaskaran K, Douglas I, Forbes H, dos-Santos-Silva I, Leon DA, Smeeth L. Body-mass index and risk of 22 specific cancers: a population-based cohort study of 5.24 million UK adults. Lancet. 2014;384(9945):755-765.
doi pubmed - Khan SS, Ning H, Wilkins JT, Allen N, Carnethon M, Berry JD, Sweis RN, et al. Association of body mass index with lifetime risk of cardiovascular disease and compression of morbidity. JAMA Cardiol. 2018;3(4):280-287.
doi pubmed - Morimoto LM, White E, Chen Z, Chlebowski RT, Hays J, Kuller L, Lopez AM, et al. Obesity, body size, and risk of postmenopausal breast cancer: the Women's Health Initiative (United States). Cancer Causes Control. 2002;13(8):741-751.
doi pubmed - Flegal KM, Kit BK, Graubard BI. Body mass index categories in observational studies of weight and risk of death. Am J Epidemiol. 2014;180(3):288-296.
doi pubmed - Obesity: preventing and managing the global epidemic. Report of a WHO consultation. World Health Organ Tech Rep Ser. 2000;894:i-xii, 1-253.
- Blair CK, Wiggins CL, Nibbe AM, Storlie CB, Prossnitz ER, Royce M, Lomo LC, et al. Obesity and survival among a cohort of breast cancer patients is partially mediated by tumor characteristics. NPJ Breast Cancer. 2019;5:33.
doi pubmed - Widschwendter P, Friedl TW, Schwentner L, DeGregorio N, Jaeger B, Schramm A, Bekes I, et al. The influence of obesity on survival in early, high-risk breast cancer: results from the randomized SUCCESS A trial. Breast Cancer Res. 2015;17(1):129.
doi pubmed - Barrett T, Wilhite SE, Ledoux P, Evangelista C, Kim IF, Tomashevsky M, Marshall KA, et al. NCBI GEO: archive for functional genomics data sets—update. Nucleic Acids Res. 2013;41(Database issue):D991-D995.
doi pubmed - Chen K, Lu P, Beeraka NM, Sukocheva OA, Madhunapantula SV, Liu J, Sinelnikov MY, et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin Cancer Biol. 2022;83:556-569.
doi pubmed - Hopkins BD, Goncalves MD, Cantley LC. Obesity and cancer mechanisms: cancer metabolism. J Clin Oncol. 2016;34(35):4277-4283.
doi pubmed - Iyengar NM, Gucalp A, Dannenberg AJ, Hudis CA. Obesity and cancer mechanisms: tumor microenvironment and inflammation. J Clin Oncol. 2016;34(35):4270-4276.
doi pubmed - Kwan ML, Kroenke CH, Sweeney C, Bernard PS, Weltzien EK, Castillo A, Factor RE, et al. Association of high obesity with PAM50 breast cancer intrinsic subtypes and gene expression. BMC Cancer. 2015;15:278.
doi pubmed - Daling JR, Malone KE, Doody DR, Johnson LG, Gralow JR, Porter PL. Relation of body mass index to tumor markers and survival among young women with invasive ductal breast carcinoma. Cancer. 2001;92(4):720-729.
doi pubmed - Beeraka NM, Doreswamy SH, Sadhu SP, Srinivasan A, Pragada RR, Madhunapantula SV, Aliev G. The role of exosomes in stemness and neurodegenerative diseases-chemoresistant-cancer therapeutics and phytochemicals. Int J Mol Sci. 2020;21(18):6818.
doi pubmed - Manogaran P, Beeraka NM, Padma VV. The Cytoprotective and Anti-cancer Potential of Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera. Curr Top Med Chem. 2019;19(32):2940-2957.
doi pubmed - Kamineni A, Anderson ML, White E, Taplin SH, Porter P, Ballard-Barbash R, Malone K, et al. Body mass index, tumor characteristics, and prognosis following diagnosis of early-stage breast cancer in a mammographically screened population. Cancer Causes Control. 2013;24(2):305-312.
doi pubmed - Fair AM, Wujcik D, Lin JM, Grau A, Wilson V, Champion V, Zheng W, et al. Obesity, gynecological factors, and abnormal mammography follow-up in minority and medically underserved women. J Womens Health (Larchmt). 2009;18(7):1033-1039.
doi pubmed - Majed B, Moreau T, Senouci K, Salmon RJ, Fourquet A, Asselain B. Is obesity an independent prognosis factor in woman breast cancer? Breast Cancer Res Treat. 2008;111(2):329-342.
doi pubmed - Jiralerspong S, Kim ES, Dong W, Feng L, Hortobagyi GN, Giordano SH. Obesity, diabetes, and survival outcomes in a large cohort of early-stage breast cancer patients. Ann Oncol. 2013;24(10):2506-2514.
doi pubmed - Ma X, Liu C, Xu X, Liu L, Gao C, Zhuang J, Li H, et al. Biomarker expression analysis in different age groups revealed age was a risk factor for breast cancer. J Cell Physiol. 2020;235(5):4268-4278.
doi pubmed - Youn HJ, Han W. A Review of the Epidemiology of Breast Cancer in Asia: Focus on Risk Factors. Asian Pac J Cancer Prev. 2020;21(4):867-880.
doi pubmed - Asif HM, Sultana S, Akhtar N, Rehman JU, Rehman RU. Prevalence, risk factors and disease knowledge of breast cancer in Pakistan. Asian Pac J Cancer Prev. 2014;15(11):4411-4416.
doi pubmed - Falkenberry SS, Legare RD. Risk factors for breast cancer. Obstet Gynecol Clin North Am. 2002;29(1):159-172.
doi pubmed - Berstad P, Coates RJ, Bernstein L, Folger SG, Malone KE, Marchbanks PA, Weiss LK, et al. A case-control study of body mass index and breast cancer risk in white and African-American women. Cancer Epidemiol Biomarkers Prev. 2010;19(6):1532-1544.
doi pubmed - White AJ, Nichols HB, Bradshaw PT, Sandler DP. Overall and central adiposity and breast cancer risk in the Sister Study. Cancer. 2015;121(20):3700-3708.
doi pubmed - Fang X, Wei J, He X, Lian J, Han D, An P, Zhou T, et al. Quantitative association between body mass index and the risk of cancer: A global Meta-analysis of prospective cohort studies. Int J Cancer. 2018;143(7):1595-1603.
doi pubmed - Michels KB, Terry KL, Willett WC. Longitudinal study on the role of body size in premenopausal breast cancer. Arch Intern Med. 2006;166(21):2395-2402.
doi pubmed - Rowland AS, Baird DD, Long S, Wegienka G, Harlow SD, Alavanja M, Sandler DP. Influence of medical conditions and lifestyle factors on the menstrual cycle. Epidemiology. 2002;13(6):668-674.
doi pubmed - Key TJ, Pike MC. The dose-effect relationship between 'unopposed' oestrogens and endometrial mitotic rate: its central role in explaining and predicting endometrial cancer risk. Br J Cancer. 1988;57(2):205-212.
doi pubmed - Key TJ, Pike MC. The role of oestrogens and progestagens in the epidemiology and prevention of breast cancer. Eur J Cancer Clin Oncol. 1988;24(1):29-43.
doi pubmed - Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ, Friedman ER, Slingerland JM. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention. CA Cancer J Clin. 2017;67(5):378-397.
doi pubmed - Neuhouser ML, Aragaki AK, Prentice RL, Manson JE, Chlebowski R, Carty CL, Ochs-Balcom HM, et al. Overweight, obesity, and postmenopausal invasive breast cancer risk: a secondary analysis of the women's health initiative randomized clinical trials. JAMA Oncol. 2015;1(5):611-621.
doi pubmed - Arnold M, Jiang L, Stefanick ML, Johnson KC, Lane DS, LeBlanc ES, Prentice R, et al. Duration of adulthood overweight, obesity, and cancer risk in the women's health initiative: a longitudinal study from the United States. PLoS Med. 2016;13(8):e1002081.
doi pubmed - Pajares B, Pollan M, Martin M, Mackey JR, Lluch A, Gavila J, Vogel C, et al. Obesity and survival in operable breast cancer patients treated with adjuvant anthracyclines and taxanes according to pathological subtypes: a pooled analysis. Breast Cancer Res. 2013;15(6):R105.
doi pubmed - Cespedes Feliciano EM, Kwan ML, Kushi LH, Chen WY, Weltzien EK, Castillo AL, Sweeney C, et al. Body mass index, PAM50 subtype, recurrence, and survival among patients with nonmetastatic breast cancer. Cancer. 2017;123(13):2535-2542.
doi pubmed - Jeon YW, Kang SH, Park MH, Lim W, Cho SH, Suh YJ. Relationship between body mass index and the expression of hormone receptors or human epidermal growth factor receptor 2 with respect to breast cancer survival. BMC Cancer. 2015;15:865.
doi pubmed - Kawai M, Tomotaki A, Miyata H, Iwamoto T, Niikura N, Anan K, Hayashi N, et al. Body mass index and survival after diagnosis of invasive breast cancer: a study based on the Japanese National Clinical Database-Breast Cancer Registry. Cancer Med. 2016;5(6):1328-1340.
doi pubmed - Mowad R, Chu QD, Li BD, Burton GV, Ampil FL, Kim RH. Does obesity have an effect on outcomes in triple-negative breast cancer? J Surg Res. 2013;184(1):253-259.
doi pubmed - Sparano JA, Wang M, Zhao F, Stearns V, Martino S, Ligibel JA, Perez EA, et al. Obesity at diagnosis is associated with inferior outcomes in hormone receptor-positive operable breast cancer. Cancer. 2012;118(23):5937-5946.
doi pubmed - Ligibel JA, Cirrincione CT, Liu M, Citron M, Ingle JN, Gradishar W, Martino S, et al. Body Mass Index, PAM50 subtype, and outcomes in node-positive breast cancer: CALGB 9741 (Alliance). J Natl Cancer Inst. 2015;107(9):djv179.
doi - Sahin S, Erdem GU, Karatas F, Aytekin A, Sever AR, Ozisik Y, Altundag K. The association between body mass index and immunohistochemical subtypes in breast cancer. Breast. 2017;32:227-236.
doi pubmed - Fontanella C, Lederer B, Gade S, Vanoppen M, Blohmer JU, Costa SD, Denkert C, et al. Impact of body mass index on neoadjuvant treatment outcome: a pooled analysis of eight prospective neoadjuvant breast cancer trials. Breast Cancer Res Treat. 2015;150(1):127-139.
doi pubmed - Ewertz M, Jensen MB, Gunnarsdottir KA, Hojris I, Jakobsen EH, Nielsen D, Stenbygaard LE, et al. Effect of obesity on prognosis after early-stage breast cancer. J Clin Oncol. 2011;29(1):25-31.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


