| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Review
Volume 14, Number 3, June 2023, pages 165-173
Pituitary Metastases From Differentiated Thyroid Cancers: A Systematic Review
Nosakhare Paul Ilerhunmwuwaa, b, Mustafa Wasifuddina, Jamal Perrya, Narek Hakobyana, Lawrence Inyanga, Zhanna Zavgorodnevaa, Lilit Gasparyana, Muhammad Tahira
aInternal Medicine Department, Brookdale Hospital Medical Center/One Brooklyn Health, Brooklyn, NY, USA
bCorresponding Author: Nosakhare Paul Ilerhunmwuwa, Internal Medicine Department, Brookdale Hospital Medical Center/One Brooklyn Health, Brooklyn, NY, USA
Manuscript submitted April 6, 2023, accepted May 12, 2023, published online June 11, 2023
Short title: PM From DTC
doi: https://doi.org/10.14740/wjon1593
| Abstract | ▴Top |
Background: Pituitary metastasis (PM) from differentiated thyroid cancer (DTC) is extremely rare and may adversely affect outcomes. We aimed to assess the characteristics and outcomes of patients with PM from DTC.
Methods: We systematically reviewed the literature on publications on PM and the different DTC histologic types (papillary, follicular, and Hurthle cell cancers). Three databases (PubMed, Embase, and Scopus) were searched for articles published from 1967 to 2022. Survival time was estimated as the period from the first treatment of PM to the time of death or last follow-up.
Results: Twenty-five articles comprising 27 cases that met the eligibility criteria were identified using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA). The median age of the patients was 60 years (23 - 86). A preponderance of females (66.7%) with PM most commonly reported papillary thyroid cancer (55.6%). This was followed by follicular thyroid cancer (37.0%) and Hurthle cell cancer (7.4%). The most common presentations were headache, nausea, and vomiting, with visual symptoms in 44.4%. Diabetes insipidus was an infrequent finding (7.4%). The median time from diagnosis or first treatment of DTC to the diagnosis of PM was 3 years (0 - 25). The most common endocrine abnormality was hyperprolactinemia (63.2%), while the most frequently deficient hormone was luteinizing hormone (50%). The most common treatment modality for PM was a combination of radiotherapy and surgery with or without radio-iodine. At the end of the follow-up, 30% of the patients died. Only 33.3% of the patients achieved complete resolution of symptoms. The overall median survival time was 12 months (3 - 108). There was a moderate inverse correlation between the age of patients and survival, which was, however, not statistically significant (rs = -0.45, P = 0.103).
Conclusion: PM from DTC is extremely rare, and Hurtle cell cancer appears to be the least associated with PM. Diabetes insipidus is a rare initial manifestation of PM from DTC. Complete resolution of symptoms is less likely to be achieved in PM from DTC. Older age may confer an increased survival tendency, probably due to more intracranial space volume in older people compared to the younger population. Larger studies are needed to examine the relationship between age and survival in PM from DTC. Also, more observational data are required to determine the predictors of survival and compare the efficacy of the different treatment modalities in patients with PM from DTC.
Keywords: Pituitary metastasis; Differentiated thyroid cancer; Papillary thyroid cancer; Follicular thyroid cancer; Hurthle cell thyroid cancer; Systematic review
| Introduction | ▴Top |
The pituitary gland is a rare site for metastases, and many reported cases arise from cancer of the breast and lung [1, 2]. The current understanding of pituitary metastasis (PM) characteristics and behaviors is restricted predominantly to case reports and a few cross-sectional studies. PM may be discovered incidentally, usually in patients with a history of cancer, or may present with symptoms of hormonal deficiencies, mass effects, and diabetes insipidus (DI) [1-4]. Some studies have identified DI as frequent in PM, especially with primary lung or breast cancer [1, 2].
PM from differentiated thyroid cancer (DTC) is extremely uncommon and represents a rare site for metastases [5-8]. Less than 30 cases have been reported in the literature [9, 10]. Some reports have indicated differences in PM manifestations in the DTC setting compared to non-thyroid cancers. For example, Barbaro et al [9] and Ilerhunmwuwa et al [10] suggested that DI was an infrequent finding in PM from DTC.
DTC constitutes more than 90% of all thyroid cancers and carries the best prognosis with a 10-year survival rate of at least 90% [11, 12]. However, outcomes may be adversely affected by the presence of metastases in DTC. There are limited data on the characteristics and outcomes of patients with PM from DTC. An understanding of the behavior of DTC in the PM setting is relevant in guiding clinical decision-making and management of these patients. We aimed to systematically review the literature for studies on PM from DTC and synthesize the available data on the characteristics, presentations, treatment modalities, and survival outcomes.
| Materials and Methods | ▴Top |
Search strategy
The Preferred Reporting Item for Systematic Review and Meta-Analysis (PRISMA) guidelines were followed in this systematic review [13]. Three electronic databases (PubMed, Embase, and Scopus) were comprehensively searched for articles on DTC and PM published until August 2022. The search was done using the following subject headings: papillary thyroid cancer, follicular thyroid cancer, Hurthle cell thyroid cancer, differentiated thyroid cancer, and pituitary metastasis. No specific search filters were applied. References were uploaded to Zotero v5.0.81 (Zotero.org). This reference manager automatically identified duplicate articles, which were removed. The remaining list of references was manually and carefully checked by two authors (NPI and JC) to ensure that all duplicate articles had been removed.
Study selection
The inclusion criteria for eligible studies were: confirmed diagnosis of DTC and PM, human subjects, and extractable data. The exclusion criteria included: 1) undifferentiated thyroid cancers; 2) medullary thyroid cancers; 3) co-existing primary cancers; 4) other cancers with metastases to the pituitary gland; 5) articles in any other language apart from English; 6) animal subjects; 7) non-extractable data; and 8) post-mortem diagnosis of PM and/or DTC.
Two authors (NPI and JC) independently selected eligible articles based on the inclusion and exclusion criteria. This was done in two stages: firstly, the titles and abstracts of the articles were screened for relevance. After that, the full texts of the relevant articles were examined to determine those that met the inclusion criteria. Articles that did not meet these criteria were excluded. Disagreements between the two authors in this process were resolved by a third author (IU).
Data extraction
The following data were extracted from each article: first author and year of publication, country, number of cases, study design, gender, age (years), histology of DTC, location of PM in relation to sella turcica, clinical presentations, cranial nerve involvement, prolactin levels, deficient anterior pituitary hormone(s), the time interval between diagnosis of DTC and development of PM, treatment modalities, and survival time. Survival time was defined as the period from the first treatment of PM to the time of death or last follow-up.
Quality assessment
The quality of each eligible 25 articles was assessed by two authors (LG and ZZ) using the tool and approach suggested by Murad et al [14]. Each domain was assigned 0 or 1 point. For domains with sub-groups, their assigned weight score (1) was divided by the number of sub-groups. A zero point was assigned to an item if the answer was negative. Three items in the causality domain (questions of other alternative causes that may explain the observation ruled out, challenge/rechallenge phenomenon, and presence of dose-response effect) were excluded due to irrelevance to our study (Supplementary Material 1, www.wjon.org). Based on this, the following grading system was used to determine the quality of each article: 0 - 2 (low quality), 3 (average quality), and 4 (good quality). Disagreements in judgment were resolved by a third author (LI).
Statistical analyses
All statistical analyses were conducted using Microsoft Excel (Professional Plus 2016, USA). Patient-level data were extracted from the studies, and all analyses were based on available cases. All variables were converted to a uniform scale of measurement. Categorical variables were presented with frequency and percentages, while continuous variables were presented with median and ranges. A Spearman rank-sum correlation was used to assess the relationship between the age of patients and survival time following PM treatment. Statistical significance was determined at P < 0.05.
| Results | ▴Top |
Literature search results
The literature review was conducted according to the PRISM guidelines, and our search produced 377 articles (Fig. 1). Duplicate publications were removed, leaving a total of 161 articles. Screening the articles by title-abstract resulted in 37 of them being for full-text review. A final total of 25 articles were obtained after meeting the outlined criteria for inclusion.
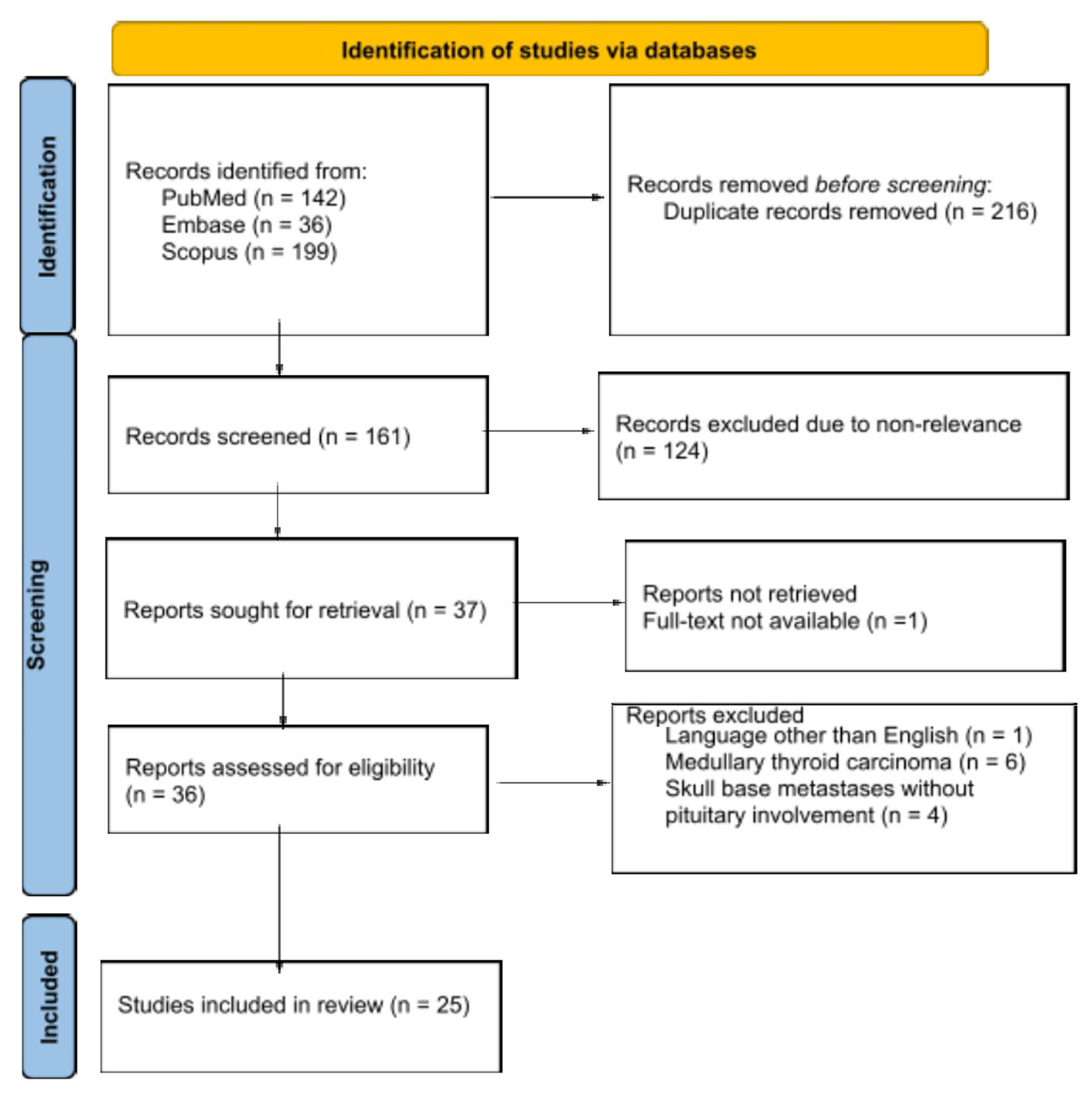 Click for large image | Figure 1. Flowchart of the systematic review. |
Quality assessment results
The results of the quality assessment of the eligible studies are shown in Supplementary Material 2 (www.wjon.org) [5, 9, 10, 15-36]. Twenty studies had moderate quality, while the remaining five were rated low.
Study overview
Twenty-five articles comprising 27 patients (Table 1) published from 1967 to 2022 were included in this systematic review [5, 9, 10, 15-36]. Of these, seven studies each were from North America (five from the United States and two from Canada), Asia and Europe, one each from South America and Australia, and two from the Middle East. Regarding the study designs, 23 were case reports, while two were case series.
 Click to view | Table 1. Demographics, Regions, and Characteristics of Patients With PM From DTC |
Baseline clinical and pathologic characteristics of study participants
Table 2 shows the baseline clinical characteristics of the study participants. The median age of the patients was 60 years (23 - 86). The proportion of females was 66.7% (n = 18) compared to males, which was 33.3% (n = 9). PM was most commonly reported with papillary thyroid cancer (PTC, 55.6%), followed by follicular thyroid cancer (37.0%), while Hurthle cell cancer (HCC) was least associated with it (7.4%). Figure 2 shows the various forms of presentation of PM in DTC. The most common initial presentations were visual symptoms with mass effects (headache, nausea/vomiting, or seizures) in 44.4% (n = 12) of cases [5, 10, 17, 18, 21, 25, 26, 29, 30, 32-34]. DI was the initial presenting feature in 7.4% of cases (n = 2), while apoplexy occurred only in 3.7% of patients (n = 1). Non-specific symptoms (loss of appetite, nausea, vomiting, weight loss, fatigue, and dizziness) occurred in 7.4% of cases. The PM was an incidental finding in 7.4% of cases. The most common cranial nerve palsy involved was the oculomotor nerve in 75% of patients (n = 6).
 Click to view | Table 2. Baseline Clinical and Pathologic Characteristics of Study Participants |
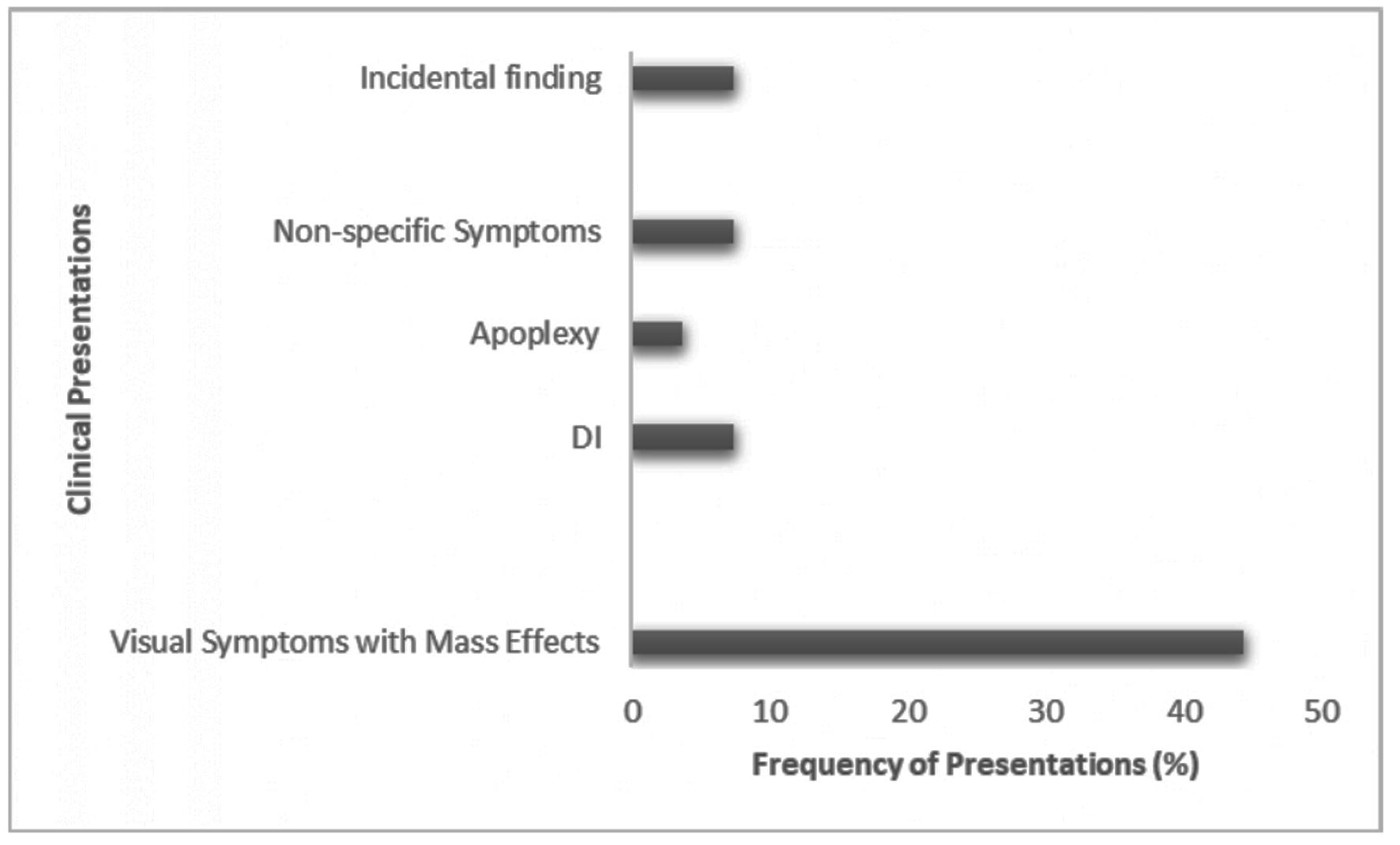 Click for large image | Figure 2. Frequency of presenting symptoms in PM from DTC. DI: diabetes insipidus; PM: pituitary metastasis; DTC: differentiated thyroid cancer. |
Sixteen studies [5, 9, 10, 18-20, 22-25, 29, 30, 33-36] reported other deficient hormones: luteinizing hormone (LH) was the most frequently deficient hormone in 50% (n = 8) of cases while growth hormone deficiency was the least common, occurring in 12.5% (n = 2) of the patients. Serum prolactin was elevated in 63.2% (n = 12) of cases [5, 9, 10, 18, 20, 21, 23, 27, 30, 31, 33, 34]. The PMs were extra-sellar lesions or extensions in 88.4% of cases (n = 23). The median time from diagnosis or first treatment of DTC to the diagnosis of PM was 3 years (0 - 25).
Intervention and outcomes
The treatment modalities for PM are shown in Table 3 [5, 9, 10, 15-36]. The overall median survival (follow-up) time was 12 months (3 - 108) from 18 studies (n = 20) which reported the survival (follow-up) time [5, 9, 10, 15, 18, 20, 23-29, 31-35]. At the end of the follow-up, 70% (n = 14) of the patients were still alive [5, 9, 10, 15, 24-28, 31-33, 35], while 30% of them (n = 6) were deceased [18, 19, 23, 24, 29, 34]. Eleven studies [5, 9, 10, 15, 17-19, 24, 26, 28, 30] reported the clinical status of the symptoms of the patients at the end of the follow-up; only 33.3% (n = 4) achieved complete resolution from symptoms while 66.7% (n = 8) still had some residual symptoms. As shown in Supplementary Material 3 (www.wjon.org), there was a moderate inverse correlation between the age of patients and survival, which was, however, not statistically significant (rs = -0.44806, P = 0.103).
 Click to view | Table 3. Treatment Modalities and Outcomes in Patients With Pituitary Metastases From Differentiated Thyroid Cancer |
| Discussion | ▴Top |
Our review’s findings indicated that PM from DTC is extremely rare. We found that the most common DTC associated with PM was PTC, and the least was HCC. The most frequent presentation was visual symptoms with mass effects. DI was an uncommon manifestation in PM from DTC. The most common hormone abnormality was hyperprolactinemia, and the most frequent anterior pituitary hormone deficiency was LH deficiency. We also found that PM adversely affected outcomes in DTC.
PMs from DTC are very rare; our search yielded 27 cases. This is consistent with several studies which examine metastatic sites of DTC. In a multicenter study that systematically reviewed 492 cases of DTC, the authors identified 25 rare metastatic sites, but none were in the pituitary gland [37]. Another retrospective cohort study examined 240 patients with DTC; 15 patients had evidence of metastases to rare sites, with two cases involving the sella turcica, but none identified in the pituitary gland [38].
Another review of unusual metastases from DTC identified only 10 cases of PM [39]. Barbaro et al [9] reported 19 cases of PM from DTC in their literature review. A retrospective study over 10 years in a large European pituitary center identified 18 cases of PM from non-thyroid primaries [1].
The median age for diagnosis of PM from DTC in our study is similar but slightly less than that reported in PM from non-thyroid cancers [1, 2]. Our study had a female preponderance identical to the sex distribution in patients with PM from non-thyroid cancers [1, 2]. PTC was the most common type of DTC associated with PM in our study; PTC is the main contributor to the rising incidence of thyroid cancers globally [40]. It is interesting to note that HCC, which has been reported to have the highest incidence of metastases among DTC, was least frequently described with PM in our review [41].
Most PM lesions in our review were extra/parasellar, and DI was an uncommon finding (7.4 %). This is in contrast to results from previous studies of non-thyroid cancers where the frequency of DI was significant (ranging from 17% to 70%) and was reported as an endocrine hallmark of PM from non-thyroid cancers [1, 2, 30]. It has been postulated that because PMs from thyroid cancers tend to be extra-sellar lesions instead of intrasellar (which primarily affects pituitary tissue and interrupts the pituitary stalk), they present with features of mass effects than endocrine manifestations resulting from disruption of the pituitary stalk [30]. However, as reported by Lithgow et al [1], most of the PMs (about 94%) from the non-thyroid cancers they examined were supra-sellar in location. Hence, why DI is uncommon in PM from DTC remains unclear.
Hyperprolactinemia was the most common endocrine abnormality, which agrees with previous reports on PM from non-thyroid cancers [2]. The most deficient hormones in our review were gonadotrophins, followed by adrenocorticotropic hormone (ACTH) and thyroid-stimulating hormone (TSH), which is consistent with findings in PM from non-thyroid cancers [1, 2]. It is unclear why gonadotrophins are the most deficient anterior pituitary hormones in PM from both thyroid and non-thyroid cancers.
Treatment modalities for PM included radiotherapy, surgery, chemotherapy, and radio-iodine in our study. The lack of randomized controlled trials poses a challenge in establishing guidelines for managing these patients. However, as reported in the literature, management should be individualized, multidisciplinary, and aimed at ameliorating presenting symptoms [1, 2].
DTC has an excellent prognosis with a 10-year survival above 90% [11, 12]. However, the presence of PM in DTC may adversely affect survival, as shown in our review, where we found a median duration of 12 months. Nevertheless, this appears slightly better than the survival in non-thyroid cancers [1, 2]. We found that complete resolution of symptoms was less likely to be achieved regardless of the high survival in these patients, contributing to increased morbidity. We found an inverse correlation (though not statistically significant) between age and survival in patients with PM from DTC. This is probably due to more intracranial space volume with advancing age than the younger population. More observational studies are needed to examine this relationship.
Our study is the first systematic review and the largest study that examines the characteristics and outcomes of PM in patients with DTC. However, limitations include the small sample size of the study attributed to the rarity of PM in DTC and restricting studies to case reports and series. Case reports and series are prone to selection and publication biases. Therefore, the general application of our findings may be limited and should be interpreted cautiously. More observational studies are required to examine further the predictors and optimal treatment modality for PM in DTC.
| Supplementary Material | ▴Top |
Suppl 1. Tool for Assessing the Quality and Risk of Bias of Case Reports and Case Series
Suppl 2. Results of Quality Assessment
Suppl 3. Correlation Between Age of Patients With Pituitary Metastases From DTC and Survival Time (rs = -0.38, P = 0.103)
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
None to declare.
Informed Consent
Not applicable.
Author Contributions
Nosakhare Ilerhunmwuwa: conceived ideal, data search, statistical analysis, and manuscript drafting. Mustafa Wasifuddin, Jamal Perry, and Narek Hakobyan: data search. Lawrence Inyang, Zhanna Zavgorodneva, and Lilit Gasparyan: drafting of manuscript: Muhammad Tahir: revision and approval of the draft.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- Lithgow K, Siqueira I, Senthil L, Chew HS, Chavda SV, Ayuk J, Toogood A, et al. Pituitary metastases: presentation and outcomes from a pituitary center over the last decade. Pituitary. 2020;23(3):258-265.
doi pubmed pmc - Schill F, Nilsson M, Olsson DS, Ragnarsson O, Berinder K, Eden Engstrom B, Dahlqvist P, et al. Pituitary metastases: a nationwide study on current characteristics with special reference to breast cancer. J Clin Endocrinol Metab. 2019;104(8):3379-3388.
doi pubmed - Habu M, Tokimura H, Hirano H, Yasuda S, Nagatomo Y, Iwai Y, Kawagishi J, et al. Pituitary metastases: current practice in Japan. J Neurosurg. 2015;123(4):998-1007.
doi pubmed - Moreno-Perez O, Peiro FM, Lopez P, Boix E, Meoro A, Serna-Candel C, Aranda FI, et al. An isolated pituitary metastasis as presentation of a differentiated hepatocellular carcinoma mimicking a nonfunctioning macroadenoma. J Endocrinol Invest. 2007;30(5):428-433.
doi pubmed - Madronio EB, Lantion-Ang FL. The tale of two tumours: an undiagnosed case of papillary thyroid carcinoma. BMJ Case Rep. 2011;2011:bcr0820114604.
doi pubmed pmc - Chiu AC, Delpassand ES, Sherman SI. Prognosis and treatment of brain metastases in thyroid carcinoma. J Clin Endocrinol Metab. 1997;82(11):3637-3642.
doi pubmed - Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89(2):574-580.
doi pubmed - Mitsuya K, Nakasu Y, Hayashi N, Deguchi S, Takahashi T, Murakami H, Naito T, et al. Palliative cerebrospinal fluid shunting for leptomeningeal metastasis-related hydrocephalus in patients with lung adenocarcinoma: A single-center retrospective study. PLoS One. 2019;14(1):e0210074.
doi pubmed pmc - Barbaro D, Desogus N, Boni G. Pituitary metastasis of thyroid cancer. Endocrine. 2013;43(3):485-493.
doi pubmed - Ilerhunmwuwa NP, Goldspring R, Page S, Gouni R. Pituitary metastases of Hurthle cell carcinoma of the thyroid. BMJ Case Rep. 2021;14(1):e239456.
doi pubmed pmc - Sherman SI. Thyroid carcinoma. Lancet. 2003;361(9356):501-511.
doi pubmed - Eustatia-Rutten CF, Corssmit EP, Biermasz NR, Pereira AM, Romijn JA, Smit JW. Survival and death causes in differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2006;91(1):313-319.
doi pubmed - Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Int J Surg. 2021;88:105906.
doi pubmed - Murad MH, Sultan S, Haffar S, Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid Based Med. 2018;23(2):60-63.
doi pubmed pmc - Aleyadeh AA, Haddad FH, AL-omari AA, Al Sarihin KK. Pituitary metastasis of follicular thyroid carcinoma: A case report and review of the literature. Rawal Medical Journal. 2012;37:61-65.
- Bell CD, Kovacs K, Horvath E, Smythe H, Asa S. Papillary carcinoma of thyroid metastatic to the pituitary gland. Arch Pathol Lab Med. 2001;125(7):935-938.
doi pubmed - Nada Bin Hareez, Mohammad Jay, Heather Lochnan. Papillary thyroid cancer with unusual late onset pituitary and orbital choroidal metastases. Case report and literature review. Journal of Clinical and Translational Endocrinology Case Reports. 2021;20(1):100081.
doi - Chikani V, Lambie D, Russell A. Pituitary metastases from papillary carcinoma of thyroid: a case report and literature review. Endocrinol Diabetes Metab Case Rep. 2013;2013:130024.
doi pubmed pmc - Chrisoulidou A, Pazaitou-Panayiotou K, Flaris N, Drimonitis A, Giavroglou I, Ginikopoulou E, Vainas I. Pituitary metastasis of follicular thyroid carcinoma. Horm Res. 2004;61(4):190-192.
doi pubmed - Estrada AJ, Sibley SD, Drake TC. Symptomatic pituitary metastases: two case reports with contrasting clinical presentations. AACE Clin Case Rep. 2019;5(5):e294-e297.
doi pubmed pmc - Gao H, Wu S, Zhang X, Xie T. Minimally invasive follicular thyroid carcinoma mimicking pituitary adenoma: a case report. Int J Clin Exp Pathol. 2019;12(10):3949-3952.
pubmed pmc - Hirayama M, Ishida A, Inoshita N, Shiramizu H, Yoshimoto H, Kato M, Tanaka S, et al. Apoplexy in sellar metastasis from papillary thyroid cancer: A case report and literature review. Surg Neurol Int. 2022;13:253.
doi pubmed pmc - Lim W, Lim DS, Chng CL, Lim AY. Thyroid carcinoma with pituitary metastases: 2 case reports and literature review. Case Rep Endocrinol. 2015;2015:252157.
doi pubmed pmc - Masiukiewicz US, Nakchbandi IA, Stewart AF, Inzucchi SE. Papillary thyroid carcinoma metastatic to the pituitary gland. Thyroid. 1999;9(10):1023-1027.
doi pubmed - Matyja E, Zielinski G, Witek P, Kaminski G, Grajkowska W, Maksymowicz M. Pituitary metastases from the oncocytic variant of follicular thyroid carcinoma: a case report and diagnostic dilemmas. Folia Neuropathol. 2013;51(3):261-268.
doi pubmed - Meltzer DE, Parnes B, Chai R. Papillary thyroid carcinoma metastasis to the pituitary: A case report. Clin Imaging. 2021;74:41-44.
doi pubmed - Muninthorn W, Singhsnaeh A, Auttara-atthakorn A, Sriphrapadang C, Hansasuta A. Pituitary metastasis of papillary thyroid carcinoma: the first case in Thailand. J Med Assoc Thai. 2021;104:140-146.
- Ochiai H, Nakano S, Goya T, Wakisaka S, Kinoshita K. Pituitary metastasis of thyroid follicular adenocarcinoma—case report. Neurol Med Chir (Tokyo). 1992;32(11):851-853.
doi pubmed - Poplawska-Kita A, Wielogorska M, Poplawski L, Siewko K, Adamska A, Szumowski P, Mysliwiec P, et al. Thyroid carcinoma with atypical metastasis to the pituitary gland and unexpected postmortal diagnosis. Endocrinol Diabetes Metab Case Rep. 2020;2020:19-0148.
doi pubmed pmc - Prodam F, Pagano L, Belcastro S, Golisano G, Busti A, Sama M, Caputo M, et al. Pituitary metastases from follicular thyroid carcinoma. Thyroid. 2010;20(7):823-830.
doi pubmed - Yilmazlar S, Kocaeli H, Cordan T. Sella turcica metastasis from follicular carcinoma of thyroid. Neurol Res. 2004;26(1):74-78.
doi pubmed - Simmons JD, Pinson TW, Donnellan KA, Harbarger CF, Pitman KT, Griswold R. A rare case of a 1.5 mm papillary microcarcinoma of the thyroid presenting with pituitary metastasis. Am Surg. 2010;76(3):336-338.
pubmed - Simon N, Quyyumi SA, Rothman JG. Follicular thyroid cancer presenting as a sellar mass: case report and review of the literature. Endocr Pract. 2004;10(1):62-66.
doi pubmed - Souza Mota J, de Sa Caldas A, de Araujo Cortes Nascimento AGP, Dos Santos Faria M, Pereira Sobral CS. Pituitary metastasis of thyroid carcinoma: a case report. Am J Case Rep. 2018;19:896-902.
doi pubmed pmc - Vianello F, Mazzarotto R, Taccaliti A, Lora O, Basso M, Servodio O, Mian C, et al. Follicular thyroid carcinoma with metastases to the pituitary causing pituitary insufficiency. Thyroid. 2011;21(8):921-925.
doi pubmed - Zheng HM, Chen YM. A case report of pituitary metastasis from thyroid carcinoma. Chinese Journal of Endocrinology and Metabolism. 2021;37(5):481-484.
doi - Madani A, Jozaghi Y, Tabah R, How J, Mitmaker E. Rare metastases of well-differentiated thyroid cancers: a systematic review. Ann Surg Oncol. 2015;22(2):460-466.
doi pubmed - Bashank N, Farghaly H, Hassanein S, Abdel-Tawab M, Wahman M, Mahmoud H. Rare sites of metastases in patients with differentiated thyroid carcinoma and added value of SPECT/CT over planar whole body radioactive iodine scan. Eur J Hybrid Imaging. 2022;6(1):34.
doi pubmed pmc - Alqahtani A. Unusual sites of metastasis of well-differentiated thyroid cancer: a systematic review. Int J Surg Res Pract. 2021;8:122.
doi - Dunlap Q, Davies L. Differentiated thyroid cancer incidence. Surgery of the Thyroid and Parathyroid Glands. 2021;174-180.e2.
doi - Hanief MR, Igali L, Grama D. Hurthle cell carcinoma: diagnostic and therapeutic implications. World J Surg Oncol. 2004;2:27.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


