| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Case Report
Volume 15, Number 1, February 2024, pages 143-148
Significance of Beta Human Chorionic Gonadotropin in Predicting Disease Progression in Uterine Leiomyosarcoma
Abdulla Alzibdeha, Issa Mohamada, Maysa Al-Hussainib, Samer Salahc, Abdalgani Jaradatd, Ramiz Abuhijliha, Fawzi Abuhijlaa, e
aDepartment of Radiation Oncology, King Hussein Cancer Center, Amman, Jordan
bDepartment of Pathology, King Hussein Cancer Center, Amman, Jordan
cDepartment of Medical Oncology, King Hussein Cancer Center, Amman, Jordan
dFaculty of Medicine, The University of Jordan, Amman, Jordan
eCorresponding Author: Fawzi Abuhijla, Department of Radiation Oncology, King Hussein Cancer Center, PO Box 1269, Amman, Jordan
Manuscript submitted October 26, 2023, accepted November 22, 2023, published online December 9, 2023
Short title: Beta-hCG and Uterine Leiomyosarcoma
doi: https://doi.org/10.14740/wjon1748
| Abstract | ▴Top |
Uterine leiomyosarcoma is a high-grade sarcoma that might be associated with dismal outcome. There are no hematological markers that can be used to follow up the recurrence and/or progression of the tumor. We present a case of a 44-year-old female, who was diagnosed with uterine leiomyosarcoma. During her management course, serum beta human chorionic gonadotropin (β-hCG) elevation was correlated with clinical and radiological disease progression on two separate occasions. This correlation should be further investigated to potentially integrate serum β-hCG as a predictive tool for clinical behavior and treatment response.
Keywords: Uterine leiomyosarcoma; Biomarker; β-hCG
| Introduction | ▴Top |
Uterine leiomyosarcoma accounts for 2-5% of uterine malignancies [1]. There are no hematological markers that can be used to follow up the recurrence and/or progression of the tumor. We present a case of a 44-year-old female, who was diagnosed with uterine leiomyosarcoma. During her management course, serum beta human chorionic gonadotropin (β-hCG) elevation was correlated with clinical and radiological disease progression on two separate occasions.
| Case Report | ▴Top |
A 44-year-old female presented with prolonged heavy vaginal bleeding. She underwent dilation and curettage (D&C), which revealed malignant spindle cells with normal endometrial tissues, consistent with uterine leiomyosarcoma. Her initial ultrasound examination at our center showed a bulky uterus, with a mass at the posterior wall with cystic component, measuring 8 × 7 cm in size. Adnexa showed a normal appearance. Speculum examination showed normal vulvar, vaginal, and cervical anatomy.
Initial imaging with magnetic resonance imaging (MRI) and computed tomography (CT) scan to the chest, abdomen and pelvis showed local disease without distant metastasis. Pelvic MRI showed a large uterine mass consistent with the known primary mass, measuring 10 × 7 × 9 cm. Serum β-hCG was ordered, and the level was within normal value (0.1 mIU/mL) prior to her mentioned radiological examinations.
According to the multidisciplinary clinic (MDC) panel decision, the patient underwent total abdominal hysterectomy with bilateral salpingo-oophorectomy on May 30, 2019. Following surgery, the MDC panel decided to continue close follow-up without offering further treatment.
Pathological examination of the initial biopsy at the time of the D&C showed proliferation of spindle cells with severe nuclear pleomorphism and tumor necrosis. The mitotic rate was estimated at 35 mitotic figures/10 high-power field (HPF). The tumor cells were positive for desmin, smooth muscle actin and h-caldesmon, while were negative for pan-cytokeratin CK-MNF. CD10 showed focal, weak staining as shown in Figure 1. Examination of the resection specimen revealed a 9.0 cm fleshy tumor, confined to the myometrium, with no evidence of infiltration to the adjacent tissue. Microscopy showed a high-grade leiomyosarcoma, with similar morphological features to the biopsy. The final pathological stage of the case was pT1bNx, FIGO IB.
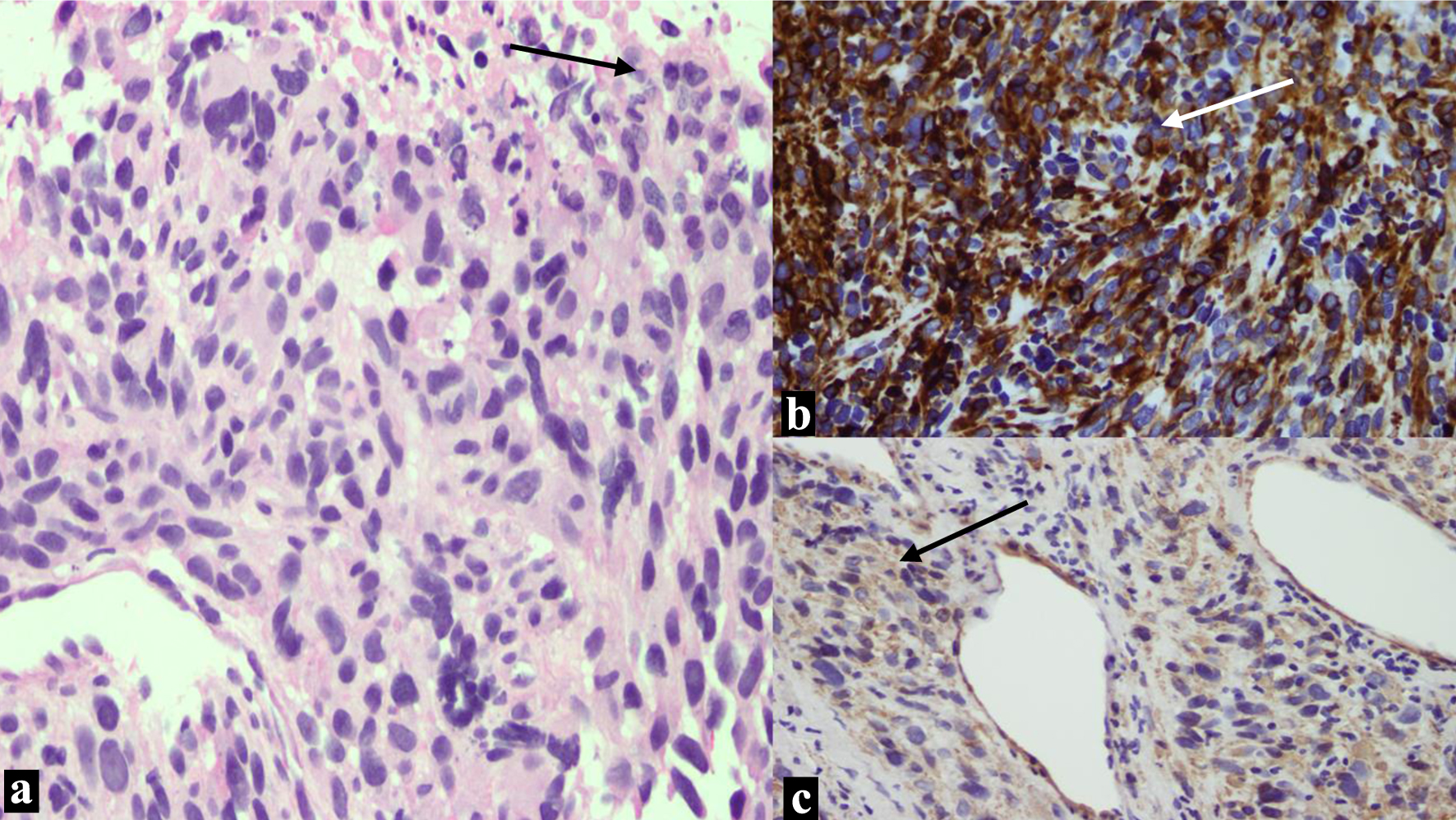 Click for large image | Figure 1. Images from the leiomyosarcoma. (a) There is proliferation of spindle cells with marked pleomorphism and necrosis at upper right corner (arrow). (b) The tumor cells are strongly diffusely positive for desmin (arrow). (c) Positive cytoplasmic staining for smooth muscle actin (arrow). |
The patient was kept on regular follow-up with imaging and Pap smears, until August 2020, when she was discovered to have an enlarging pulmonary nodule in the right upper lobe, which on subsequent imaging showed interval enlargement, along with new multiple bilateral pulmonary nodules indicating pulmonary metastases (Fig. 2). Additionally, prominent right hilar lymph nodes were noted. Furthermore, a lytic bony lesion involving the second right rib, accompanied by an associated soft tissue component was consistent with a metastatic deposit. In addition, there was a small lytic lesion involving the left seventh rib. Positron emission tomography (PET)/CT scan confirmed the widespread metastatic disease (Fig. 3). β-hCG level was repeated then and was still within normal levels (0.111 mIU/mL). A biopsy from the right sacral mass confirmed the presence of metastatic leiomyosarcoma.
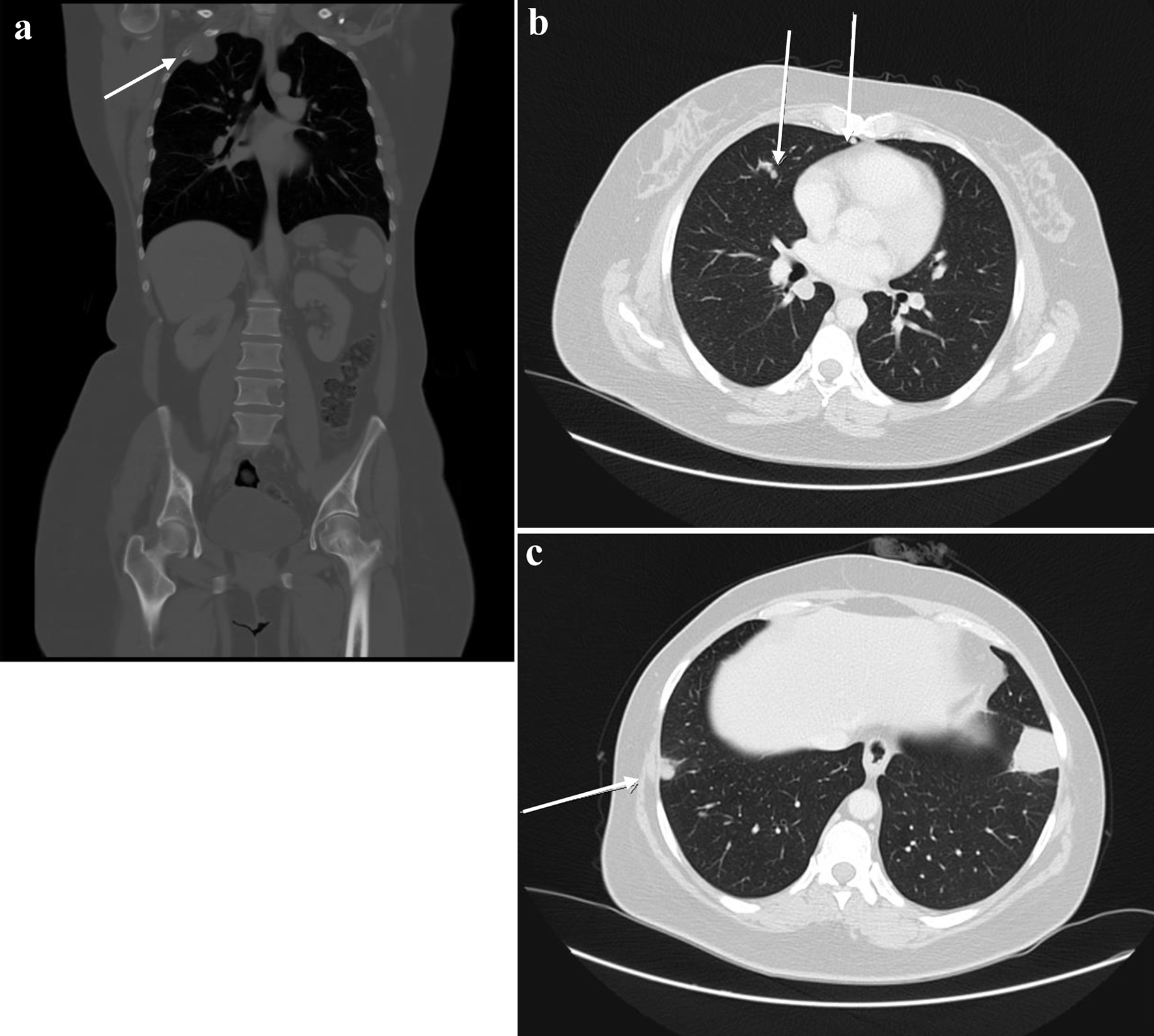 Click for large image | Figure 2. First disease progression shown on CT scan with contrast, manifested with multiple bilateral pulmonary nodules and two prominent right hilar lymph nodes, with multiple scattered destructive lytic bony lesions (arrows) (August 12, 2020) (a: coronal, bone window; b, c: axial, lung window). CT: computed tomography. |
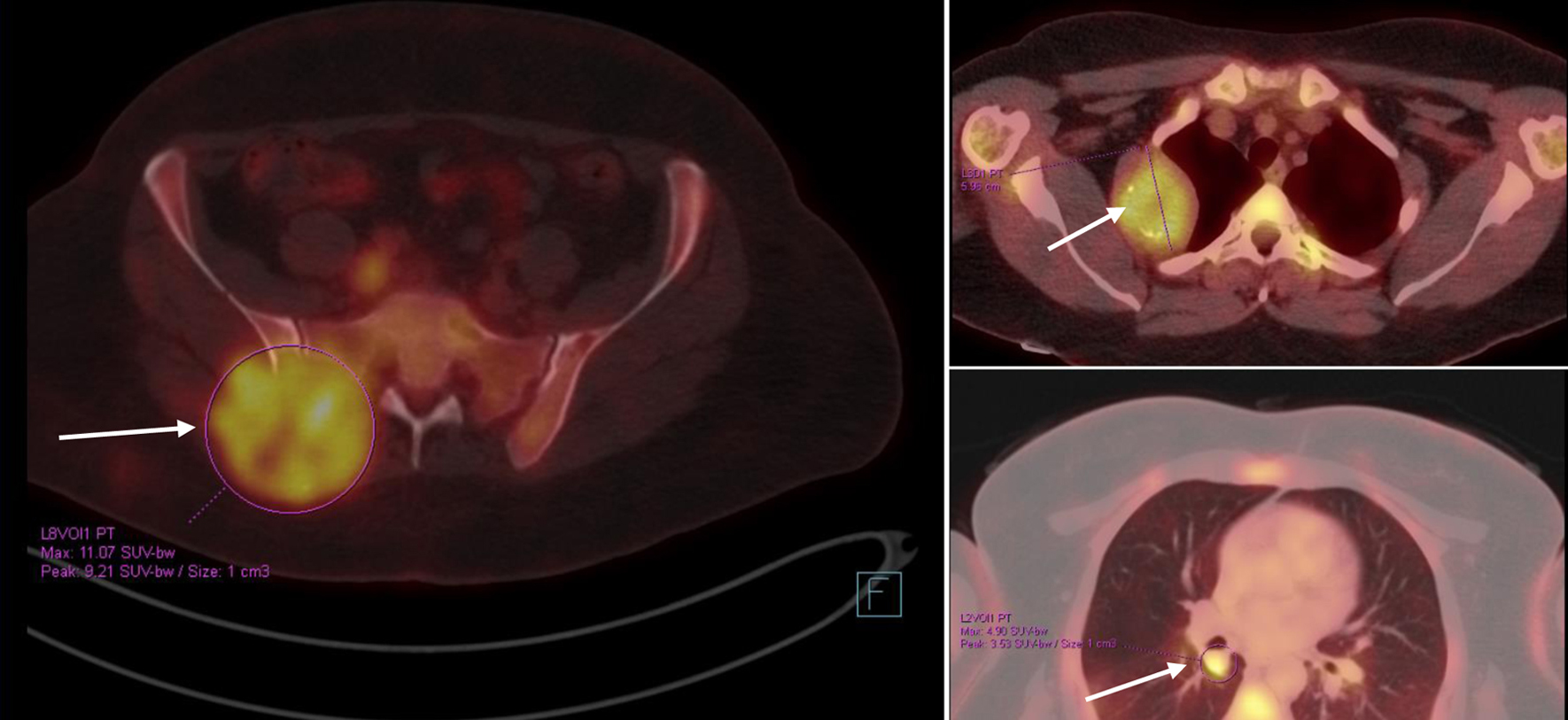 Click for large image | Figure 3. PET/CT scan showing widespread metastatic disease (arrows) (September 10, 2020). Beta-hCG level was within normal levels. Beta-hCG: beta human chorionic gonadotropin; CT: computed tomography; PET: positron emission tomography. |
The MDC panel decision was to proceed with palliative chemotherapy. On March 14, 2021, a CT scan showed disease progression in the lung and bony metastases, so she was switched to second line chemotherapy of doxorubicin, the first cycle of which was on April 26, 2021. A CT scan on June 24, 2021 revealed a larger soft tissue component in vertebral metastases, with further posterior wedging of L5 vertebral body with retro bulging compressing the thecal sac. Accordingly, she received 20 Gy/5 Fx, completed on July 12, 2021. The patient was switched to ifosfamide and received her first elective cycle on August 27, 2021, with mixed response.
On April 21, 2022, pelvic MRI showed marked progression of the metastatic deposit involving the left ischial tuberosity and acetabulum. The patient was started on pazopanib from the private sector in July 2022.
Imaging in December 2022 showed progression of the size of pulmonary metastasis, and new solitary subcapsular segment VI hepatic metastasis. The results were considered as oligo-progression, for which she received liver stereotactic body radiation therapy (SBRT) at a dose of 45 Gy/5 Fx, finished on January 3, 2023.
Imaging in February 2023 showed marked disease progression, manifested with enlarging extraosseous soft tissue component associated with destructive bony lesion involving the right second rib and left acetabulum, enlarging multifocal bilateral metastatic pulmonary lesions, and right-sided pleural effusion with pleural nodular thickening, suspicious for pleural carcinomatosis (Fig. 4).
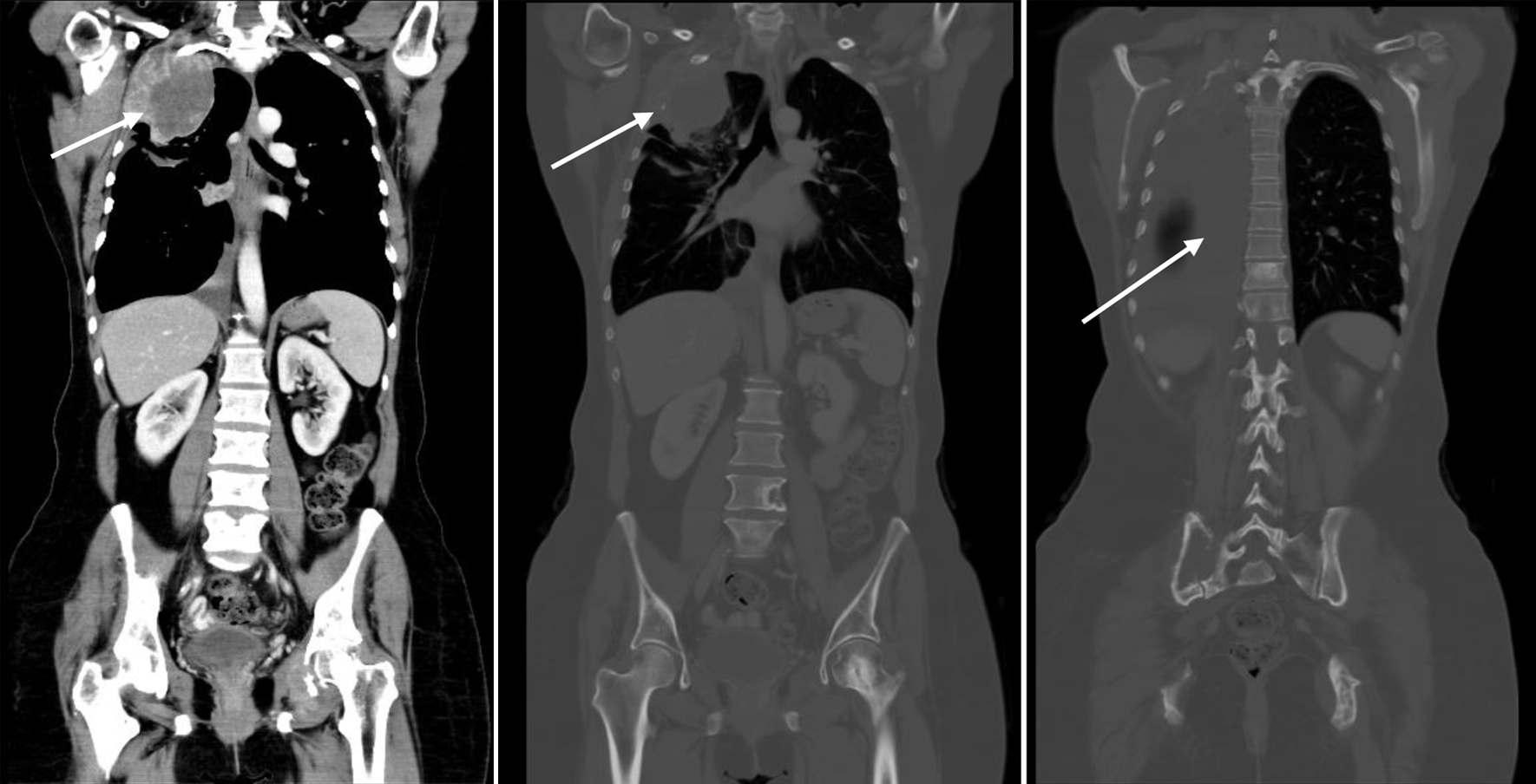 Click for large image | Figure 4. CT scan showing disease progression (February 9, 2023): enlarging extraosseous soft tissue component associated with destructive bony lesion involving the right second rib and left acetabulum, enlarging bilateral pulmonary lesions, and moderate right-sided pleural effusion with new right pleural nodular thickening, suspicious for pleural carcinomatosis (arrows). Beta-hCG level was elevated (8.43 mIU/mL). Beta-hCG: beta human chorionic gonadotropin; CT: computed tomography. |
β-hCG level was incidentally found to be mildly elevated on February 7, 2023 (8.43 mIU/mL). Repeating the test on March 12, 2023, the level remained elevated (8.44 mIU/mL). Brain MRI was done with no evidence of intracranial space occupying lesion or abnormal contrast enhancement.
Unfortunately, her disease continued to progress, and during 2023, she has undergone multiple palliative radiation treatments to various sites, and was admitted with acute hypoxic respiratory failure, pain crisis, and upper gastrointestinal bleeding.
| Discussion | ▴Top |
Identifying tumor markers can be helpful in aiding in the diagnosis, stratification of patients and assessing treatment response. Other than in pregnancy, β-hCG has been described to be produced in gestational trophoblastic disease, and by other non-trophoblastic cancers, including, transitional cell carcinoma (TCC) of the bladder and urinary tract, rectal and gastrointestinal cancers, prostate cancer, lung cancer, neuroendocrine tumors and some breast and gynecological tumors [2-9].
Blood inflammatory markers were shown to be useful in differentiation between leiomyosarcoma and leiomyoma including high white blood cell (WBC) count, absolute neutrophil count (ANC), C-reactive protein (CRP), lactate dehydrogenase (LDH), and neutrophil-to-lymphocyte ratio (NLR) levels [10].
In our case, a repeat test was performed to rule out the possibility of a false-positive result. Pregnancy was definitively excluded as the cause of elevated β-hCG levels since the patient had previously undergone hysterectomy and oophorectomy. A PET/CT scan helped in excluding any lesions indicative of an alternative primary cancer, and a brain MRI was performed to eliminate other potential secondary causes.
In the available literature, there are a few documented cases of β-hCG-producing sarcomas. β-hCG production was described in a small number of primary and recurrent osteosarcomas and osteogenic sarcomas [11-15], phyllodes tumors [16], dedifferentiated liposarcomas [17, 18] and synovial sarcomas [19]. In extra-uterine cases, elevated β-hCG was described in retroperitoneal leiomyosarcoma [18, 20, 21], spermatic cord leiomyosarcoma [22, 23], and leiomyosarcoma of the small bowel [24]. To our knowledge, our case is the fourth in literature to describe a case of uterine leiomyosarcoma associated with elevated β-hCG [25-27]. What is special about this case was that the initial β-hCG levels were within normal levels, which then showed β-hCG elevation in association with the metastatic disease, flare up and resistance to chemotherapy, and palliative radiotherapy, suggesting that β-hCG can potentially play a prognostic role in such patients.
In uterine leiomyosarcoma, the initial rise in β-hCG levels might be associated with the tumor’s aggressive histology [25]. As our case developed β-hCG elevation with metastatic disease resistance to treatment, this can suggest that the metastatic clones flaring up the disease might have undergone a process of dedifferentiation and encompassing further genetic mutations associated with treatment resistance.
Limitations of our study include that β-hCG was not measured frequently during the course of the patient’s disease, as it has no routine proven clinical value or justification to be ordered. Also, thorough pathological studies were not done on the metastatic lesions of the patient, which cannot be compared with the primary tumor and prove for sure that dedifferentiation occurred. However, it is quite evident that the disease became more resistant to treatment, which alludes to the fact that the metastatic cells harbored different genetic make-up, as described in previous researches about discrepancy in mutations between primary tumor and metastatic lesions [28]. Also, β-hCG immunohistochemistry was not done on the primary disease specimens.
Conclusion
Tumor markers that are readily measurable in most institutions, like β-hCG, are needed in tumors like uterine leiomyosarcoma, a highly aggressive and often fatal cancer. In this report, we presented a rare case of a β-hCG-producing uterine leiomyosarcoma, wherein β-hCG levels showed the potential of serving as a prognostic factor and a marker for chemotherapy treatment resistance. However, further research is needed to gain a deeper understanding of this hypothesis and validate the potential of using β-hCG production as a marker of dedifferentiation and disease progression.
Acknowledgments
None to declare.
Financial Disclosure
None to declare.
Conflict of Interest
All authors declare no conflict of interest.
Informed Consent
The authors certify that they have obtained all appropriate patient consent forms.
Author Contributions
AZ and FA designed the overall concept and outline of the manuscript; AZ and FA contributed data collection; AZ, IM, MA, SS, AJ, RA and FA contributed to the writing, review of literature, editing the manuscript and final approval of the manuscript.
Data Availability
The authors declare that data supporting the findings of this study are available within the article.
| References | ▴Top |
- George S, Serrano C, Hensley ML, Ray-Coquard I. Soft tissue and uterine leiomyosarcoma. J Clin Oncol. 2018;36(2):144-150.
doi pubmed pmc - Stenman UH, Alfthan H, Hotakainen K. Human chorionic gonadotropin in cancer. Clin Biochem. 2004;37(7):549-561.
doi pubmed - Span PN, Thomas CM, Heuvel JJ, Bosch RR, Schalken JA, vd Locht L, Mensink EJ, et al. Analysis of expression of chorionic gonadotrophin transcripts in prostate cancer by quantitative Taqman and a modified molecular beacon RT-PCR. J Endocrinol. 2002;172(3):489-495.
doi pubmed - Hotakainen K, Ljungberg B, Haglund C, Nordling S, Paju A, Stenman UH. Expression of the free beta-subunit of human chorionic gonadotropin in renal cell carcinoma: prognostic study on tissue and serum. Int J Cancer. 2003;104(5):631-635.
doi pubmed - Carpelan-Holmstrom M, Louhimo J, Stenman UH, Alfthan H, Jarvinen H, Haglund C. Estimating the probability of cancer with several tumor markers in patients with colorectal disease. Oncology. 2004;66(4):296-302.
doi pubmed - Oberg K, Wide L. hCG and hCG subunits as tumour markers in patients with endocrine pancreatic tumours and carcinoids. Acta Endocrinol (Copenh). 1981;98(2):256-260.
doi pubmed - Bepler G, Jaques G, Oie HK, Gazdar AF. Human chorionic gonadotropin and related glycoprotein hormones in lung cancer cell lines. Cancer Lett. 1991;58(1-2):145-150.
doi pubmed - Demirtas E, Krishnamurthy S, Tulandi T. Elevated serum beta-human chorionic gonadotropin in nonpregnant conditions. Obstet Gynecol Surv. 2007;62(10):675-679.quiz 691.
doi pubmed - Moutzouris G, Yannopoulos D, Barbatis C, Zaharof A, Theodorou C. Is beta-human chorionic gonadotrophin production by transitional cell carcinoma of the bladder a marker of aggressive disease and resistance to radiotherapy? Br J Urol. 1993;72(6):907-909.
doi pubmed - Suh DS, Song YJ, Roh HJ, Lee SH, Jeong DH, Lee TH, Choi KU, et al. Preoperative blood inflammatory markers for the differentiation of uterine leiomyosarcoma from leiomyoma. Cancer Manag Res. 2021;13:5001-5011.
doi pubmed pmc - Kalra JK, Mir R, Kahn LB, Wessely Z, Shah AB. Osteogenic sarcoma producing human chorionic gonadotrophin. Case report with immunohistochemical studies. Cancer. 1984;53(10):2125-2128.
doi pubmed - Lee AF, Pawel BR, Sullivan LM. Significant immunohistochemical expression of human chorionic gonadotropin in high-grade osteosarcoma is rare, but may be associated with clinically elevated serum levels. Pediatr Dev Pathol. 2014;17(4):278-285.
doi pubmed - Boss DS, Glen H, Beijnen JH, de Jong D, Wanders J, Evans TR, Schellens JH. Serum beta-HCG and CA-125 as tumor markers in a patient with osteosarcoma: case report. Tumori. 2011;97(1):109-114.
doi pubmed - Leidinger B, Bielack S, Koehler G, Vieth V, Winkelmann W, Gosheger G. High level of beta-hCG simulating pregnancy in recurrent osteosarcoma: case report and review of literature. J Cancer Res Clin Oncol. 2004;130(6):357-361.
doi pubmed - Oshrine BR, Sullivan LM, Balamuth NJ. Ectopic production of beta-hCG by osteosarcoma: a case report and review of the literature. J Pediatr Hematol Oncol. 2014;36(3):e202-206.
doi pubmed - Fisher K, Rojas K, Zelkowitz C, Borgen P, Kiss L, Zeng J. Beta-HCG-producing phyllodes tumor. Breast J. 2020;26(3):547-549.
doi pubmed - Maryamchik E, Lyapichev KA, Halliday B, Rosenberg AE. Dedifferentiated liposarcoma with rhabdomyosarcomatous differentiation producing hCG: a case report of a diagnostic pitfall. Int J Surg Pathol. 2018;26(5):448-452.
doi pubmed - Russell MJ, Flynt FL, Harroff AL, Fadare O. Dedifferentiated liposarcoma of the retroperitoneum with extensive leiomyosarcomatous differentiation and beta-human chorionic gonadotropin production. Sarcoma. 2008;2008:658090.
doi pubmed pmc - Stevens EE, Aquino J, Barrow N, Lee YC. Ectopic production of human chorionic gonadotropin by synovial sarcoma of the hip. Obstet Gynecol. 2013;121(2 Pt 2 Suppl 1):468-471.
doi pubmed - Froehner M, Gaertner HJ, Manseck A, Oehlschlaeger S, Wirth MP. Retroperitoneal leiomyosarcoma associated with an elevated beta-hCG serum level mimicking extragonadal germ cell tumor. Sarcoma. 2000;4(4):179-181.
doi pubmed pmc - Mansi IA, Ashley I, Glezerov V. Retroperitoneal leiomyosarcoma and enlarged epididymis associated with a positive pregnancy test. Am J Med Sci. 2002;324(2):104-105.
doi pubmed - Ou SM, Lee SS, Peng YJ, Sheu LF, Yao NS, Chang SY. Production of beta-hCG by spermatic cord leiomyosarcoma: a paraneoplastic syndrome? J Androl. 2006;27(5):643-644.
doi pubmed - Seidl C, Lippert C, Grouls V, Jellinghaus W. [Leiomyosarcoma of the spermatic cord with paraneoplastic beta-hCG production]. Pathologe. 1998;19(2):146-150.
doi pubmed - Meredith RF, Wagman LD, Piper JA, Mills AS, Neifeld JP. Beta-chain human chorionic gonadotropin-producing leiomyosarcoma of the small intestine. Cancer. 1986;58(1):131-135.
doi pubmed - Krishnan V, Sauthier P, Provencher D, Rahimi K. Pleomorphic undifferentiated uterine sarcoma in a young patient presenting with elevated beta-hCG and rare variants of benign leiomyoma: a case report and review of the literature. Int J Gynecol Pathol. 2020;39(4):362-366.
doi pubmed - Tsakos E, Xydias EM, Ziogas AC, Bimpa K, Sioutas A, Zarampouka K, Tampakoudis G. Uterine malignant leiomyosarcoma associated with high levels of serum beta-human chorionic gonadotropin: a case report. Clin Case Rep. 2022;10(9):e6322.
doi pubmed pmc - Di Luigi G, D'Alfonso A, Patacchiola F, Di Stefano L, Palermo P, Carta G. Leiomyosarcoma: a rare malignant transformation of a uterine leiomyoma. Eur J Gynaecol Oncol. 2015;36(1):84-87.
pubmed - Fidler IJ. Metastasis: quantitative analysis of distribution and fate of tumor emboli labeled with 125 I-5-iodo-2'-deoxyuridine. J Natl Cancer Inst. 1970;45(4):773-782.
pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


