| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 5, October 2024, pages 769-776
Prognostic Implications of Timing of Immunotherapy in Stage IV Non-Small Cell Lung Cancer
Jorge Raul Vazquez-Urrutiaa, Max Greenberga, Junjia Zhub , Shinkichi Takamoric
, Takefumi Komiyad, e
aDepartment of Medicine, Penn State Health Milton S. Hershey Medical Center, Hershey, PA 17033, USA
bDepartment of Public Health Sciences, Penn State College of Medicine, Hershey, PA 17033, USA
cDepartment of Thoracic and Breast Surgery, Oita University Faculty of Medicine, Oita, Japan
dDivision of Hematology Oncology, Penn State Cancer Institute, Hershey, PA 17033, USA
eCorresponding Author: Takefumi Komiya, Division of Hematology and Oncology, Penn State Cancer Institute, Hershey, PA 17033, USA
Manuscript submitted June 28, 2024, accepted July 23, 2024, published online August 10, 2024
Short title: Timing of Immunotherapy in Lung Cancer
doi: https://doi.org/10.14740/wjon1924
| Abstract | ▴Top |
Background: Currently, the established approach for addressing stage IV non-small cell lung cancer (NSCLC) involves combining chemotherapy with immunotherapy. However, the necessity for molecular analysis prior to commencing immunotherapy often results in a delay in its initiation following the commencement of chemotherapy. Therefore, this study aimed to study the significance of postponing immunotherapy on pertinent patient outcomes.
Methods: Using the National Cancer Database (NCBD), patients diagnosed with stage IV NSCLC between 2017 and 2018 were screened. Inclusion criteria comprised those treated with multi-agent chemotherapy as the first-line therapy within 30 days of treatment, surviving beyond 2 months of diagnosis, and absence of neuroendocrine pathology. Patients were grouped among those receiving immunotherapy within 30 days of chemotherapy, immunotherapy within 31 - 60 days of chemotherapy, or chemotherapy alone. Clinical characteristics were collected and their correlation with the timing of immunotherapy was evaluated. The impact of delaying immunotherapy on overall survival (OS) was investigated using Kaplan-Meier analysis. Multivariate Cox regression analysis was employed to identify independent prognostic variables associated with OS.
Results: Our cohort comprised 99,008 patients with clinical stage IV NSCLC diagnosed between 2017 and 2018, which were distributed in the three treatment groups described above. Patients receiving immunotherapy within 30 days of chemotherapy showed greater OS in contrast to both those subjected to delayed immunotherapy (hazard ratio (HR) = 0.74, 95% confidence interval (CI): 0.64 - 0.87, P = 0.0003). Subsequent multivariate regression analysis showed that postponing immunotherapy, older age, male sex, white race, non-adenocarcinoma histology, higher clinical N stage, use of radiation treatment, and presence of liver metastasis were all associated with worse OS.
Conclusions: Introducing immunotherapy within the first 30 days of chemotherapy initiation significantly increases survival in patients with stage IV NSCLC.
Keywords: Non-small cell lung cancer; Immunotherapy; Combination therapy
| Introduction | ▴Top |
Lung cancer remains a burden for global medicine given its high prevalence and lethality worldwide, being the leading cause of cancer mortality in males and the second in females; in addition, it is more common in men than females [1]. In the USA, there has been a steady decline of lung cancer incidence and mortality in the last decades, especially in the male population, mainly due to advances in therapy and screening strategies [2].
Once diagnosed, lung cancer classification depends on a combination of morphological, immunohistochemical and molecular analysis of the tumor according to the World Health Organization (WHO) classification [3]. Once analyzed, lung cancer is divided into two major groups, small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC), with the latter including other subgroups such as adenocarcinoma, squamous cell, and large cell carcinoma [4].
Excluding oncogene-driven disease, a combination of cytotoxic chemotherapy and immune checkpoint inhibitor (i.e., anti- programmed cell death 1 (PD1)/programmed cell death ligand 1 (PDL1) inhibitors) became a standard management in stage IV NSCLC in 2017 according to the National Comprehensive Cancer Network (NCCN) guidelines (version 2.2024). Cases with a positive driver oncogene such as EGFR NSCLC, however, need to be treated with rather appropriate targeted therapy. In routine practice, a lengthy turnaround time of molecular analysis to rule out oncogenes makes the chemoimmunotherapy combination a challenge.
In addition, unexpected toxicities when immunotherapy and targeted therapy are combined or sequenced have been reported [5-7]. Because of these restrictions many oncologists often initiate traditional chemotherapy alone for the first few cycles until a molecular result becomes available so that they can modify the first-line therapy accordingly. This practice pattern to delay initiation of immunotherapy may lead to inferior cancer outcome.
Keynote-189, an international phase III trial defining the role of additive pembrolizumab to first-line chemotherapy, demonstrated an advantage in overall survival (OS) of chemoimmunotherapy combination over chemotherapy alone regardless of PDL1 status. In the arm of chemotherapy alone, approximately 40% of patients received immunotherapy as second-line therapy. Despite the cross-over of the arms, this study showed a statistically significant difference in OS, suggesting the importance of early exposure to immunotherapy. Impact of delayed administration of immunotherapy in first-line therapy is unknown, which constitutes the main purpose of this study.
| Materials and Methods | ▴Top |
The National Cancer Database (NCDB) is a joint project of the Commission on Cancer (CoC) of the American College of Surgeons and the American Cancer Society. The CoC’s NCDB and the hospitals participating in the CoC NCDB are the sources of the deidentified data used herein; however, they have not verified the statistical validity of the data analysis, or the conclusions derived by the authors, and they are not responsible for them. The data are considered hospital-based rather than population-based [8]. Of note, this study was reviewed by the Institutional Review Board at Penn State Health and was designated exempt from human subject research.
Patients with stage IV NSCLC who were diagnosed between 2017 and 2018 were screened (n = 99,008). Those treated with multi-agent chemotherapy as a first course of therapy were included. Those with neuroendocrine pathology were excluded. In addition, patients treated with multi-agent chemotherapy within 30 days of diagnosis were included; those who were exposed to immunotherapy prior to chemotherapy were also excluded. Patients who survived less than 60 days of diagnosis were excluded as well (Fig. 1).
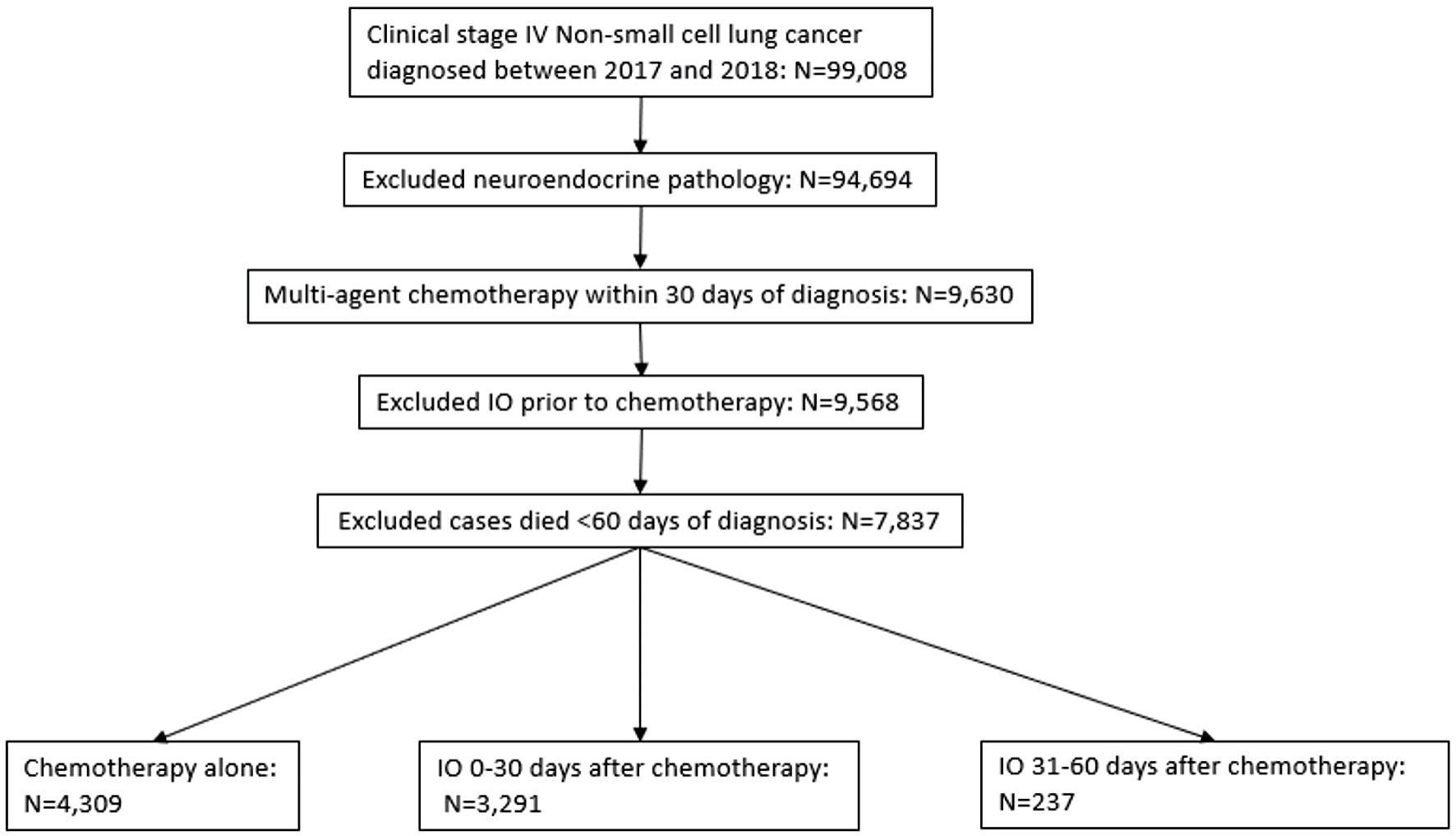 Click for large image | Figure 1. Selection criteria according to CONSORT diagram. Deidentified cases were released from the National Cancer Database. IO: immunotherapy. |
After applying initial inclusion and exclusion criteria, eligible patients were then assigned to a group of: 1) those who received early immunotherapy within 30 days of chemotherapy; 2) delayed immunotherapy within 31 - 60 days of chemotherapy; or 3) chemotherapy alone.
The main outcome variable of this study is OS, which is defined as the length of time from the initial diagnosis to death of any causes or last follow-up. Key clinical characteristics were obtained and examined within each group. These included age (< 70 versus 70 or more), sex (male versus female), race (White versus others), institution (academic versus others), Charlson-Deyo comorbidity score, year of diagnosis (2017 versus 2018), cell type (adenocarcinoma not otherwise specified versus others), clinical T stage (cT3-4 versus other), clinical N stage (cN2-N3 versus others), clinical M stage (cM1BC versus other), surgery (yes versus no), radiation (yes versus no), presence of brain and liver metastasis (yes versus no). A propensity score matching (PSM) analysis was conducted to compare groups 1) and 2).
Statistical methods
The associations between clinical demographics and timing of immunotherapy were examined using Chi-square tests. Kaplan-Meier survival curves were generated and compared between timing of immunotherapy groups using log-rank test. Univariable and multivariable Cox proportional hazard regression models were used to calculate hazard ratios (HRs) and their 95% confidence intervals (CIs) for survival since time of tissue diagnosis, and independent prognostic factors were identified. In the analysis of patients with stage IV NSCLC who received immunotherapy within 0 - 30 days and 31 - 60 of chemotherapy, PSM was performed to reduce bias in our selected sample. The following variables were used in PSM: age, sex, race, surgery, radiation, liver metastasis, clinical M stage, clinical N stage, and histology. PSM was performed using “MatchIt” package version 4.5.5 running on R version 4.4.0 (R Foundation for Statistical Computing, Vienna, Austria). One-to-one nearest neighbor matching was done with propensity scores estimated through logistic regression. All other analyses were performed using JMP® 14.0 (SAS Institute Inc., Cary, NC, USA). All tests are two-sided, and P < 0.05 was considered statistically significant.
| Results | ▴Top |
Patient characteristics
Our sample selection process is described in Figure 1. The study included 99,008 patients diagnosed with clinical stage IV NSCLC between 2017 and 2018. After applying exclusion criteria, 7,837 patients were assigned for group distribution (Fig. 1). Among these, 3,291 received immunotherapy (0 - 30 days from diagnosis), 237 received delayed immunotherapy (31 - 60 days from diagnosis), and 4,309 received chemotherapy only. Patients’ characteristics and the associations between immunotherapy use and clinical factors are presented in Table 1. Characteristics associated with immunotherapy use were male sex, age less than 70, White race, histology of adenocarcinoma, clinical T stage (other than T3-4), clinical M stage (cM1BC), and no radiation therapy.
 Click to view | Table 1. Clinical Characteristics of Eligible Cases (N (%)) |
We successfully matched 237 patients in group 1 with the patients in group 2 using propensity score. The characteristics after PSM were shown here (Supplementary Material 1, www.wjon.org). No significant differences in patient characteristics were found between the matched patients in groups 1 and 2.
Early immunotherapy within 30 days of initiation of chemotherapy prolongs OS among NSCLC patients
A total of 3291,237, and 4,309 were selected for groups 1, 2, 3 for the analysis, respectively. Among these groups, median OS was 14.5, 9.5, and 10.0 months for the group 1, 2, and 3, respectively. A statistically significant difference in OS was observed between groups 1 and 2 (HR = 0.74, 95% CI: 0.64 - 0.87, P = 0.0003) (Table 2, Fig. 2). There was no statistically significant difference in OS between groups 2 and 3. The significant OS difference between groups 1 and 2 was confirmed by PSM analysis (n = 237 each, HR = 0.73, 95% CI: 0.59 - 0.90, P = 0.004) (Fig. 3).
 Click to view | Table 2. Univariate and Multivariable Cox Regression Analyses for Overall Survival in Patients With Stage IV NSCLC Treated With Combination Therapy (Within 30 Days vs. 30 - 61 Days) |
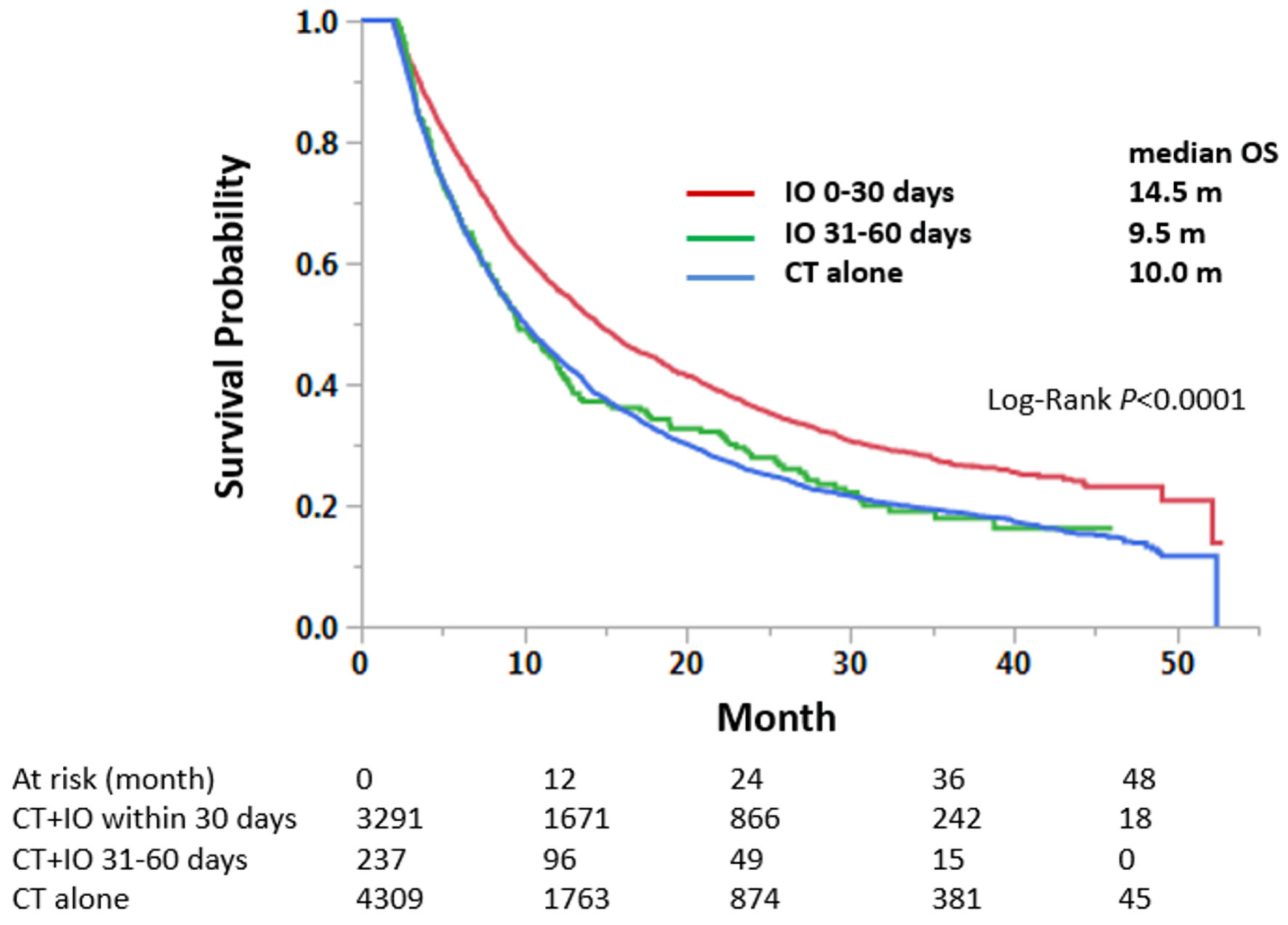 Click for large image | Figure 2. Immunotherapy within the first 30 days of chemotherapy treatment improves overall survival in patients with stage IV non-small cell lung cancer, compared to delayed or no immunotherapy statuses. Median survival months and log-rank P values are shown. CT: chemotherapy; IO: immunotherapy; OS: overall survival. |
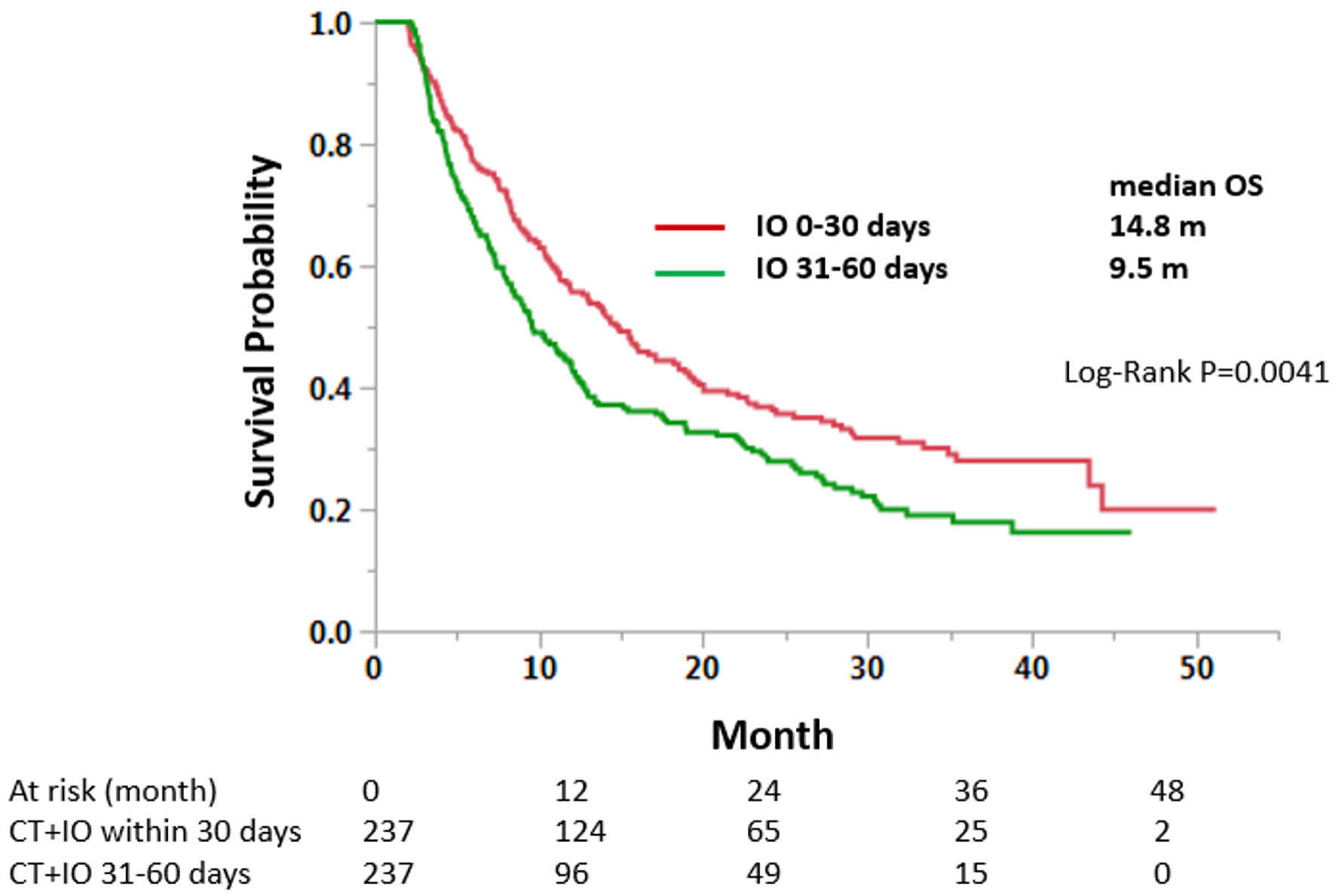 Click for large image | Figure 3. Propensity score matching analysis of early (30 days) and delayed (31 - 60 days) immunotherapy groups. Matched cases were compared for OS. CT: chemotherapy; IO: immunotherapy; OS: overall survival. |
Furthermore, multivariate Cox regression analysis demonstrated that group 1 had a significantly prolonged OS over group 2, and that immunotherapy use in relation to chemotherapy was an independent predictor of increased OS whereas delayed immunotherapy was associated with decreased OS (Table 2). This relation persisted after PSM analysis (Supplementary Material 2, www.wjon.org). In addition, age higher than 70, male sex, White race, non-adenocarcinoma histology, N2-3 stage, use of radiotherapy and presence of liver metastasis were all independent predictors of poor OS in this group of patients (Table 2). After PSM analysis, only male sex, non-adenocarcinoma histology and the presence of liver metastasis remained as independent predictors (Supplementary Material 2, www.wjon.org).
| Discussion | ▴Top |
To our knowledge, this is the first study evaluating the prognostic implications of the timing of immunotherapy in combination with chemotherapy in patients with stage IV NSCLC. We demonstrated that early introduction of immunotherapy within 30 days of starting chemotherapy was associated with a significantly increased OS, which was further validated on multivariate and PSM analyses. This finding could be the result of chemotherapy-mediated enhancement of the immune response to cancerous cells, by modifying their structure so they are more detectable to the immune system, while simultaneously decreasing cancer-mediated immunomodulation [9]. The addition of immunotherapy to further potentiate this response would translate to better clinical outcomes.
To further support this, several clinical trials have demonstrated significant improvement in several outcomes, including survival for patients with NSCLC when combining immunotherapy and chemotherapy. One of the most relevant trials in this regard is, as mentioned previously, the KEYNOTE-189 study, which investigated the efficacy of pembrolizumab in combination with standard chemotherapy for patients with metastatic non-squamous NSCLC, successfully demonstrating substantial increase in OS and progression-free survival (PFS) in patients with combination therapy. Moreover, this combination did not increase the frequency of adverse events attributed to chemotherapy or immunotherapy alone [10]. Another notable trial is the CheckMate-227 study, which randomized patients with stage IV or recurrent NSCLC to receive either a combination of nivolumab plus ipilimumab, nivolumab with chemotherapy or chemotherapy alone in patients. The results showed greater OS, treatment response, and PFS with the combination immunotherapy and immunotherapy plus chemotherapy regimens compared to chemotherapy alone [11]. Furthermore, there are ongoing trials evaluating whether combining immunotherapy and chemotherapy as front-line therapy after diagnosis is superior to using both treatment strategies separately, like the EA5163/S1709 INSIGNA trial [12], which indirectly matches the intervention evaluated in this study.
It is certainly important to acknowledge some of the limitations inherent in our study. Firstly, the non-randomized assignment of patients to treatment groups, and the retrospective nature of our analysis introduce inherent biases. To mitigate these, PSM and multivariate analyses were employed.
Furthermore, while the NCDB database serves as a valuable resource for cancer-related research, it does not include all the relevant clinical data, such as Eastern Cooperative Oncology Group (ECOG) performance status, disease-free survival, molecular status (e.g., EGFR mutation), details on immunotherapy-related adverse events, and concurrent non-cancer medications. Of note, the absence of molecular profiling data could potentially constrain the generalizability of our findings, particularly given the paramount importance of targeted therapies for individuals harboring driver oncogenes such as EGFR and ALK. However, it warrants acknowledgment that the representation of such subgroups within the NCDB cohort is relatively modest, constituting approximately 20% of participants, with a predominant demographic skew towards Caucasians.
Additionally, our findings lack external validation from independent cohorts, demonstrating the need for further validation to strengthen the robustness of our results. Moving forward, prospective studies incorporating a more comprehensive array of variables are warranted to enhance the evidential support for our findings.
In conclusion, this retrospective study using one of the largest cancer databases suggests that delaying immunotherapy when used in combination with chemotherapy have detrimental effects on survival outcome and that early introduction of immunotherapy correlates with favorable clinical outcomes.
| Supplementary Material | ▴Top |
Suppl 1. Characteristic of patients after propensity score matching.
Suppl 2. Univariate and multivariable Cox regression analyses for overall survival in patients with stage IV NSCLC treated with combination therapy: within 30 days vs. 30 - 61 days (propensity score matching analysis).
Acknowledgments
We thank Dr. Patrick Ma and Kristen Derr for administrative assistance.
Financial Disclosure
Takefumi Komiya received advisory fees from Novocure and Regenerone, and institutional research funding from Gilead.
Conflict of Interest
Takefumi Komiya received advisory fees from Novocure and Regenerone. The other authors declared no financial interest.
Informed Consent
Not applicable.
Author Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Takefumi Komiya. The first draft of the manuscript was written by Jorge Raul Vazquez-Urrutia, and all authors read and approved the final manuscript.
Data Availability
The datasets analyzed during the current study are available via NCDB upon request.
| References | ▴Top |
- Leiter A, Veluswamy RR, Wisnivesky JP. The global burden of lung cancer: current status and future trends. Nat Rev Clin Oncol. 2023;20(9):624-639.
doi pubmed - Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74(3):229-263.
doi pubmed - Nicholson AG, Tsao MS, Beasley MB, Borczuk AC, Brambilla E, Cooper WA, Dacic S, et al. The 2021 WHO classification of lung tumors: impact of advances since 2015. J Thorac Oncol. 2022;17(3):362-387.
doi pubmed - Rodriguez-Canales J, Parra-Cuentas E, Wistuba II. Diagnosis and molecular classification of lung cancer. Cancer Treat Res. 2016;170:25-46.
doi pubmed - Schoenfeld AJ, Arbour KC, Rizvi H, Iqbal AN, Gadgeel SM, Girshman J, Kris MG, et al. Severe immune-related adverse events are common with sequential PD-(L)1 blockade and osimertinib. Ann Oncol. 2019;30(5):839-844.
doi pubmed pmc - McMahon DJ, McLaughlin R, Naidoo J. Is immunotherapy beneficial in patients with oncogene-addicted non-small cell lung cancers? A narrative review. Cancers (Basel). 2024;16(3):527.
doi pubmed pmc - Spigel DR, Reynolds C, Waterhouse D, Garon EB, Chandler J, Babu S, Thurmes P, et al. Phase 1/2 study of the safety and tolerability of nivolumab plus Crizotinib for the first-line treatment of anaplastic lymphoma kinase translocation - positive advanced non-small cell lung cancer (CheckMate 370). J Thorac Oncol. 2018;13(5):682-688.
doi pubmed - American College of Surgeons. National Cancer Data-base. https://www.facs.org/quality-programs/cancer-programs/national-cancer-database/. Accessed April 30, 2024.
- Bracci L, Schiavoni G, Sistigu A, Belardelli F. Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ. 2014;21(1):15-25.
doi pubmed pmc - Gandhi L, Rodriguez-Abreu D, Gadgeel S, Esteban E, Felip E, De Angelis F, Domine M, et al. Pembrolizumab plus Chemotherapy in Metastatic Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(22):2078-2092.
doi pubmed - Hellmann MD, Paz-Ares L, Bernabe Caro R, Zurawski B, Kim SW, Carcereny Costa E, Park K, et al. Nivolumab plus Ipilimumab in Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2019;381(21):2020-2031.
doi pubmed - EA5163/S1709 INSIGNA: a randomized, phase III study of firstline immunotherapy alone or in combination with chemotherapy in induction/maintenance or postprogression in advanced nonsquamous Non-Small Cell Lung Cancer (NSCLC) with immunobiomarker SIGNature-driven analysis. Identifier NCT03793179. U.S. National Library of Medicine. 2024. https://www.clinicaltrials.gov/study/NCT03793179.
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


