| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 4, August 2023, pages 246-254
High Expression of F-Box Protein 43 Is Associated With Poor Prognosis and Adjuvant Chemotherapy Resistance in Colorectal Cancer
Junyu Liua, e, Xi Yangb, e, Miao Lic, e, Ying Ying Liud, Yulan Wangd, Shichao Lid, f, Fengping Zhenga, f
aDepartment of Gastroenterology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, China
bDepartment of Medical Service, General Hospital of Xinjiang Military Command, Urumqi, Xinjiang, China
cSchool of Rehabilitation Medicine, Xinjiang Medical University, Urumqi, Xinjiang, China
dDepatment of Pathology, General Hospital of Xinjiang Military Command, Urumqi, Xinjiang, China
eThese authors contributed equally to this work.
fCorresponding Author: Fengping Zheng, Department of Gastroenterology, The Third Affiliated Hospital of Sun Yat-Sen University, Guangzhou, Guangdong 510630, China; Shichao Li, Department of Pathology, General Hospital of Xinjiang Military Command, Urumqi, Xinjiang 830000, China
Manuscript submitted June 22, 2023, accepted June 30, 2023, published online August 4, 2023
Short title: Prognostic Value of FBXO43 in Colorectal Cancer
doi: https://doi.org/10.14740/wjon1642
| Abstract | ▴Top |
Background: The F-box protein 43 (FBXO43), also referred to as endogenous meiotic inhibitor 2 (EMI2), has been linked to the advancement of various types of cancer, such as hepatocellular carcinoma, breast cancer, cholangiocarcinoma, and gastric cancer. Nevertheless, the precise function of FBXO43 in colorectal cancer (CRC) remains unclear. This study employed data from The Cancer Genome Atlas (TCGA) and clinical specimens to analyze the expression, prognostic value, and chemotherapeutic advantages of FBXO43 in CRC.
Methods: Level 3 RNA sequencing data pertaining to 631 cases of colon and rectal adenocarcinomas (COAD-READ) were downloaded from TCGA. The data were utilized to analyze the expression, prognosis, and related signal pathways of FBXO43. The expression of FBXO43 in clinical samples was subsequently confirmed through the use of real-time quantitative polymerase chain reaction (qPCR) and immunohistochemistry (IHC). Lastly, a tissue microarray (TMA) consisting of 120 cases of CRC and corresponding normal tissues was established to investigate the relationship between FBXO43 and survival outcomes.
Results: Results from both the TCGA analysis and clinical samples indicated that FBXO43 was significantly upregulated in CRC tissues in comparison to normal tissues. Moreover, high level of FBXO43 was found to be relevant to malignant clinical features, such as differentiation, lymph node metastasis, and pathological stage, as well as unfavorable prognosis in CRC patients. Subgroup analysis further demonstrated that FBXO43 could be an effective parameter for stratifying low-risk CRC patients. Notably, survival analysis showed that patients with high level of FBXO43 had worse overall survival (OS) and disease-free survival (DFS) following adjuvant chemotherapy, and FBXO43 was distinctly upregulated in chemotherapy-resistant patients’ primary CRC tissues.
Conclusions: FBXO43 was upregulated and associated with poor prognosis of CRC; patients with high expression of FBXO43 may not be benefit from adjuvant chemotherapy.
Keywords: FBXO43; Prognosis; Chemotherapy; Colorectal cancer
| Introduction | ▴Top |
Colorectal cancer (CRC) leads to approximately 10% of cancer-related deaths worldwide and ranks second among women and third among men [1]. The estimated 5-year and 10-year survival rates are 65% and 58%, respectively, with men having 25% higher incidence and mortality rates than women [2]. The initiation of CRC is induced by a range of factors [3], such as age, race, gender, body weight, diet, smoking, alcohol consumption, genetic epidemiology, and inflammatory bowel disease, etc. The prognosis for patients with CRC remains unfavorable due to frequent late-stage diagnoses. However, the implementation of widespread CRC screening has facilitated the early detection of precancerous adenomas, resulting in a significant reduction in both the incidence and mortality rates of CRC [4, 5]. Despite the advent of immunotherapy, surgical resection and chemotherapy remain the primary modalities for treating CRC, with adjuvant chemotherapy typically recommended for high-risk patients with stage II or III CRC following surgery. As such, the identification of novel biomarkers or therapeutic targets for CRC screening is of utmost importance.
The F-box protein 43 (FBXO43), also referred to as endogenous meiotic inhibitor 2 (EMI2), is a member of the F-box protein family and is distinguished by an F-box motif of approximately 40 amino acids. In spermatocytes, FBXO43 impedes UBE2C (ubiquitin conjugating enzyme E2C) binding to anaphase-promoting complex/cyclosome (APC/C) and facilitates meiotic MII (the second metaphase of meiosis) arrest in the early diplotene stage of prophase I [6]. Moreover, various studies have indicated the involvement of FBXO43 in the development of tumors. Xu et al [7] conducted a gene coexpression network analysis, revealing that FBXO43, along with nine other genes, could potentially serve as biomarkers for predicting survival of patients with hepatocellular carcinoma (HCC). This finding was subsequently validated in clinical HCC tissues and cell lines [8, 9]. FBXO43 has been observed to be overexpressed in breast cancer and cholangiocarcinoma [10, 11]. Nevertheless, the precise function of FBXO43 in CRC remains unclear. Consequently, additional investigation is warranted to elucidate the relationship between FBXO43 and CRC pathogenesis and prognosis.
In this study, we identified that the upregulated expression of FBXO43 was linked to unfavorable prognosis in CRC. A correlation was observed between the expression of FBXO43 and various clinicopathologic features as well as patients’ prognosis. Moreover, survival analysis showed that patients with high level of FBXO43 had worse overall survival (OS) and disease-free survival (DFS) following postoperative chemotherapy, and FBXO43 was upregulated in patients who were resistant to chemotherapy. These data suggested that FBXO43 could serve as a potential biomarker for chemotherapy.
| Materials and Methods | ▴Top |
Patients and samples
The aforementioned studies were granted approval by the Ethical Committee of the General Hospital of Xinjiang Military Command. A cohort of 120 CRC tumor and corresponding para-tumor normal tissues were procured from patients who underwent surgical resection at the General Hospital of the Xinjiang Military Command (Urumqi, China) between 2011 and 2018. Comprehensive details are provided here (Supplementary Material 1, www.wjon.org). The Kaplan-Meier method was employed to analyze the OS and DFS outcomes. During the final follow-up appointment, the OS was calculated as the duration between the date of surgery and the date of death or censoring, whereas the DFS was determined by utilizing the date of surgery and the date of recurrence or censoring. FOBX43 mRNA expression was analyzed in 30 paired CRC and para-tumor tissues from CRC patients through real-time polymerase chain reaction (qPCR). This study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
RNA extract and real-time quantitative polymerase chain reaction (qPCR) analysis
RNA extraction from tissues was performed using Trizol (Invitrogen), followed by reverse transcription using a Reverse Transcription System (Promega). The resulting cDNA was combined with SYBR Green PCR Kit (Roche) and specific primers to initiate real-time qPCR using the Roche Light Cycler 96 System (Roche, USA). The relative mRNA expression levels were determined using the 2-ΔΔCt method and normalized to β-actin (FBXO43, forward primer: 5'-GGAAAGTAAGCAGAAATTGGCGTG-3', reverse primer: 5'-GAGTGGCAGCATCCTCGACATT-3'; β-actin, forward primer: 5'-GACGATATCGCTGCGCTGG-3', reverse primer: 5'-CCACGATGGAGGGGAATA-3').
Immunohistochemistry (IHC)
Tissue samples were formalin-fixed and paraffin-embedded and sectioned for IHC staining as described previously [12]. Tissue microarray (TMA) was blocked by 3% hydrogen peroxide and goat serum and then incubated with primary antibody (anti-FBXO43) and second secondary antibody (goat anti-rabbit). Finally, positive granules were stained by DAB (K3486, DAKO), followed by hematoxylin counterstaining. The stained sections were scanned by ScanScope XT (Aperio Technologies) scanner, and two experienced pathologists independently reviewed the image of section. Finally, Image Scope (Aperio Technologies) algorithms of positive pixel count were used to quantify and score the images.
TCGA data preparation
Level 3 of RNA sequencing data with clinical information of 631 cases of CRC (COAD-READ) and 57 cases of normal tissue were downloaded from the TCGA website [13] to investigate the expression and prognostic potential of FBXO43 by R (version 4.2.1).
Differentially expressed genes (DEGs) and enrichment analysis
The “EdgeR” R package was employed to investigate the DEGs between high (70-100%) and low (0-30%) expression of FBXO43 among CRC samples from TCGA. The results were visualized using a volcano plot. Subsequently, DEGs with |log2FC| > 1 and adjusted P < 0.05 were selected for Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis using the “clusterProfiler” package. Additionally, all DEGs were subjected to Gene Set Enrichment Analysis (GSEA) using the same package.
Statistical analysis
Statistical analysis was performed using R software version 4.2.1. The two-group comparison was conducted using either the Student’s t-test or Fisher exact test. Pearson and Spearman correlation were employed to determine correlation. The Kaplan-Meier method was used to compare patients’ survival of distinct subgroups, and log-rank analysis was conducted to test the significance of the results. A P value smaller than 0.05 was considered statistically significant.
| Results | ▴Top |
FBXO43 was highly expressed in CRC tissues
The expression of FBXO43 was initially evaluated in TCGA samples and clinical tissues. Results of TCGA data (Fig. 1a, b) demonstrated a significant upregulation of FBXO43 in CRC tissues (n = 631) compared to normal tissues (n = 57). Furthermore, qPCR analysis revealed an upregulation of FBXO43 mRNA in CRC tissues (n = 30) (Fig. 1c). Additionally, IHC analysis confirmed an apparent increase in the protein level of FBXO43 in CRC tissues (Fig. 1d). Furthermore, within cohort 1 consisting of 120 patients, it was observed that 56.67% of patients displayed elevated expression of FBXO43 in CRC tissues compared to corresponding para-tumor tissues (Fig. 1e). These findings indicate a significant upregulation of FBXO43 in CRC tissues.
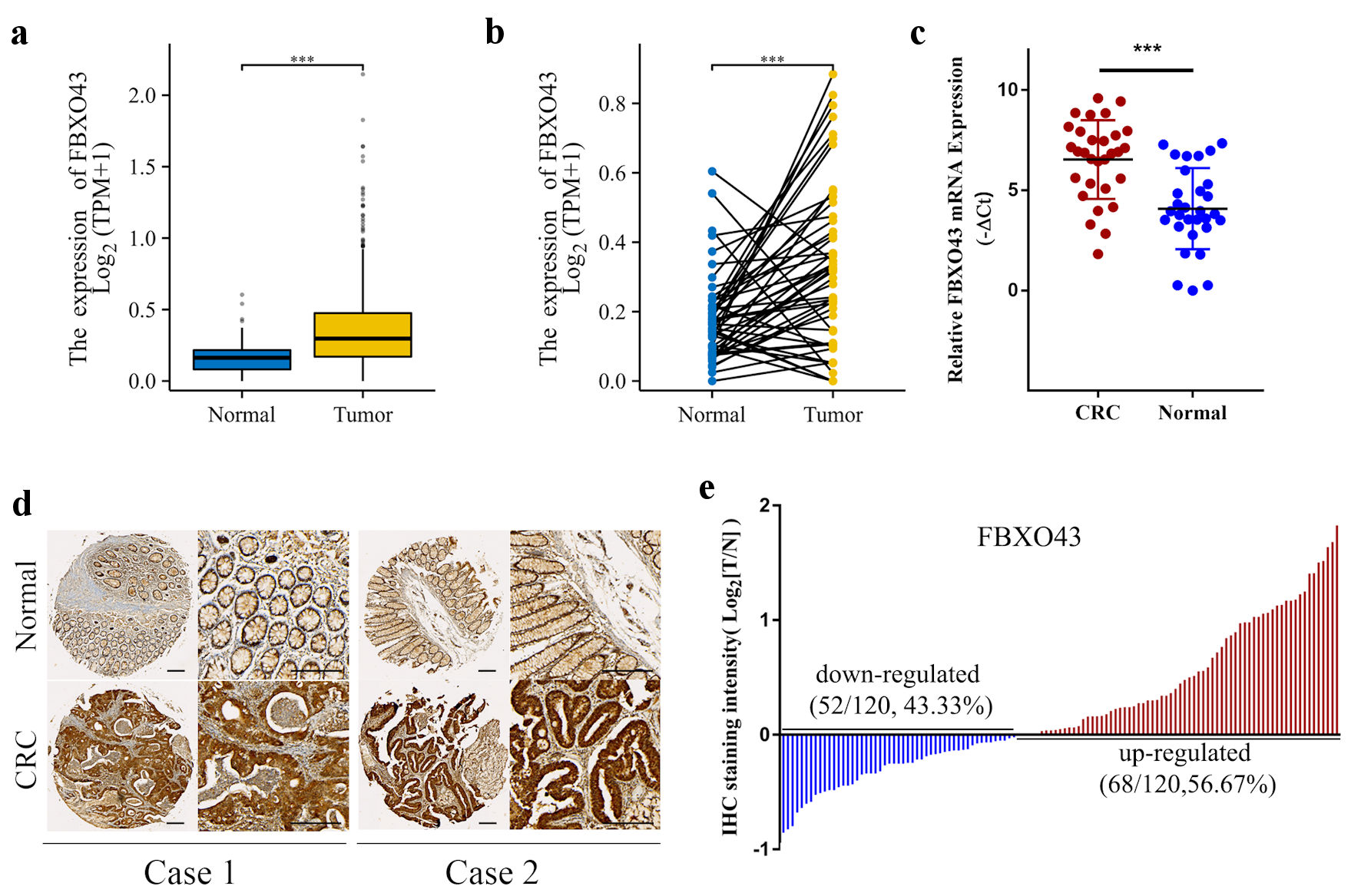 Click for large image | Figure 1. FBXO43 was highly expressed in CRC tissues. (a, b) The expression of FBXO43 in unpaired (a) and pair (b) tissues between CRC and normal tissues in TCGA. (c) mRNA expression of FBXO43 in 30 pairs of CRC and para-tumor normal tissues were determined by real-time qPCR. (d) Representative IHC staining of FBXO43 expression in CRC (scale bar: 100 µm). (e) FBXO43 was upregulated in the majority of CRC patients (56.67%). FBXO43: F-box protein 43; CRC: colorectal cancer; TCGA: The Cancer Genome Atlas; PCR: polymerase chain reaction; IHC: immunohistochemistry. |
High FBXO43 was associated with poor clinical characteristics and prognosis of CRC
To further evaluate the clinical significance of FBXO43 in CRC, 120 CRC patients were stratified into “FBXO43 high” and “FBXO43 low” groups according to the median FBXO43 level quantified by Image Scope. Correlation analysis demonstrated a positive association between elevated FBXO43 protein levels and aggressive clinical features, such as T stage, N stage, and pathological stage (Supplementary Material 1, www.wjon.org). Moreover, patients with poorly differentiated tumors, lymph node metastasis, or advanced pathological stages exhibited pronounced FBXO43 staining (Fig. 2a-c). Additionally, the Kaplan-Meier survival analysis revealed that elevated FBXO43 levels in CRC were linked to inferior OS and DFS (Fig. 2d, e). The results of the univariate analysis indicated a significant correlation between elevated levels of FBXO43 and unfavorable patient outcomes. Furthermore, the multivariate COX regression analysis revealed that high expression of FBXO43 was an independent prognostic factor for OS in patients. Although patients with high FBXO43 exhibited a slightly inferior DFS, the difference was not statistically significant (Supplementary Material 2, 3, www.wjon.org). Survival analysis also revealed the prognostic value of FBXO43 in the TCGA cohort (Supplementary Material 4, www.wjon.org).
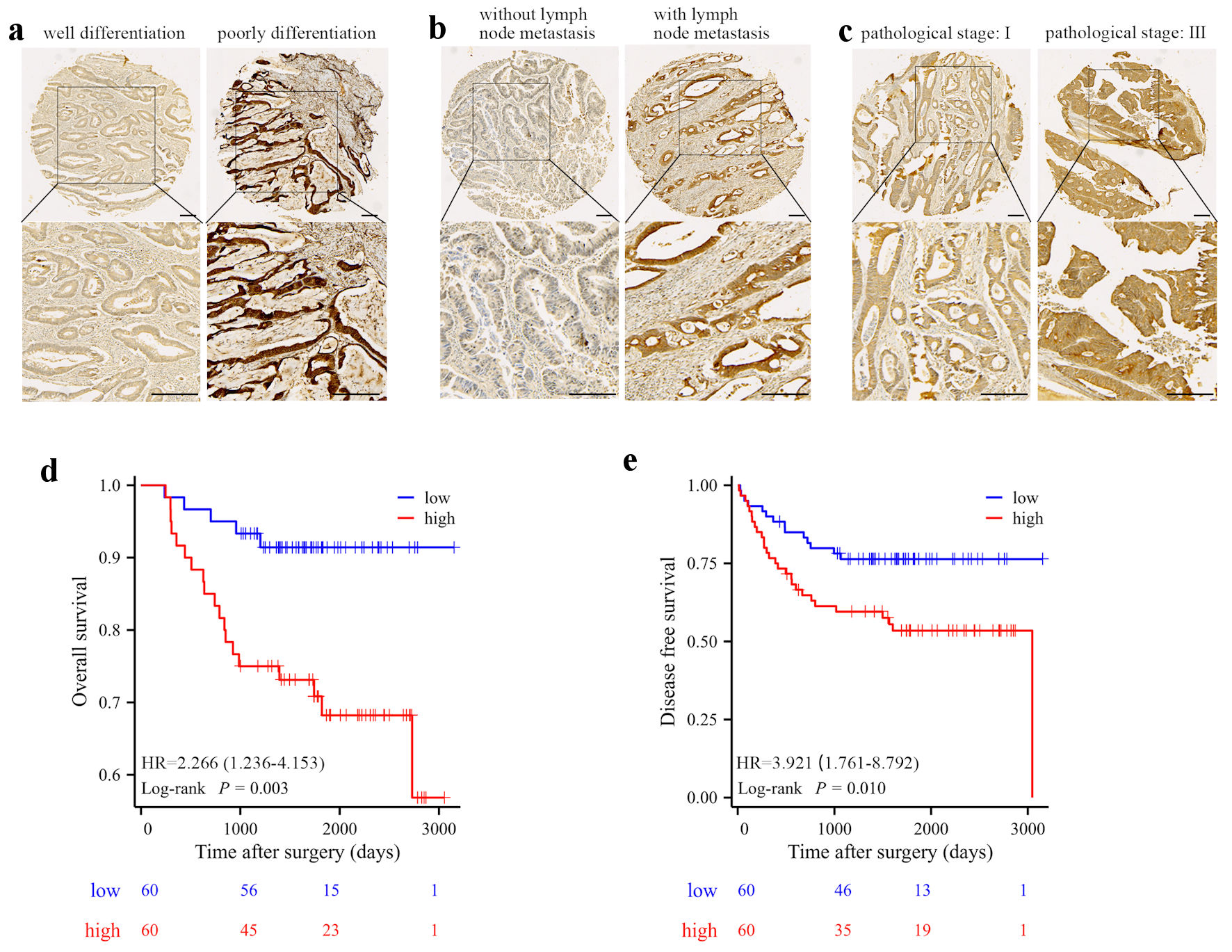 Click for large image | Figure 2. High level of FBXO43 was associated with poor clinical characteristics and prognosis. (a-c) Representative IHC staining of FBXO43 expression in different clinical stage CRCs: differentiation (a), lymph metastasis (b), and pathological stage (c) (scale bar: 100 µm). (d-e) OS (d) and RFS (e) of CRC patients after surgery resection were compared between the “high FBXO43” and “low FBXO43” groups using Kaplan-Meier analysis. FBXO43: F-box protein 43; IHC: immunohistochemistry; CRC: colorectal cancer; OS: overall survival; DFS: disease-free survival; HR: hazard ratio. |
Prognostic value of FBXO43 for subgroup stratification of CRC
Given that age, gender, tumor stage, tumor size, and invasion are widely acknowledged factors associated with prognosis, the prognostic significance of FBXO43 across various clinical subgroups was investigated through univariate Cox regression. The results showed that patients with high FBXO43 levels had significant worse OS in several subgroup including female (P = 0.011, hazard ratio (HR) = 7.140 (1.562 - 32.633)), age ≥ 60 (P = 0.022, HR = 3.802 (1.211 - 11.933)), tumor size < 5 cm (P = 0.014, HR = 4.756 (1.379 - 16.403)), moderate differentiation (P = 0.012, HR = 6.675 (1.505 - 29.606)), no vessel invasion (P = 0.012, HR = 4.066 (1.356 - 12.199)), no nerve invasion (P = 0.008, HR = 4.341 (1.457 - 12.932)), M stage 0 (P = 0.013, HR = 3.560 (1.310 - 9.676)) and T stage III - IV (P = 0.042, HR = 3.114 (1.039 - 9.333)) (Fig. 3a). While worse DFS were observed in subgroup patients with high FBXO43 levels including female (P = 0.01, HR = 3.121 (1.318 - 7.390)), age < 59 (P = 0.045, HR = 3.610 (1.027 - 12.687)), tumor size < 5 cm (P = 0.016, HR = 2.872 (1.213 - 6.802)), moderate differentiation (P = 0.002, HR = 4.646 (1.731 - 12.469)), no vessel invasion (P = 0.002, HR = 3.445 (1.546 - 7.676)), no nerve invasion (P = 0.011, HR = 2.431 (1.226 - 4.821)) and M stage 0 (P = 0.022, HR = 2.134 (1.114 - 4.089)) (Fig. 3b). Additionally, Kaplan-Meier survival analysis revealed consistent prognostic value in those subgroups (Fig. 3c-j). These data suggested that FBXO43 could be an effective parameter for stratifying low-risk CRC patients.
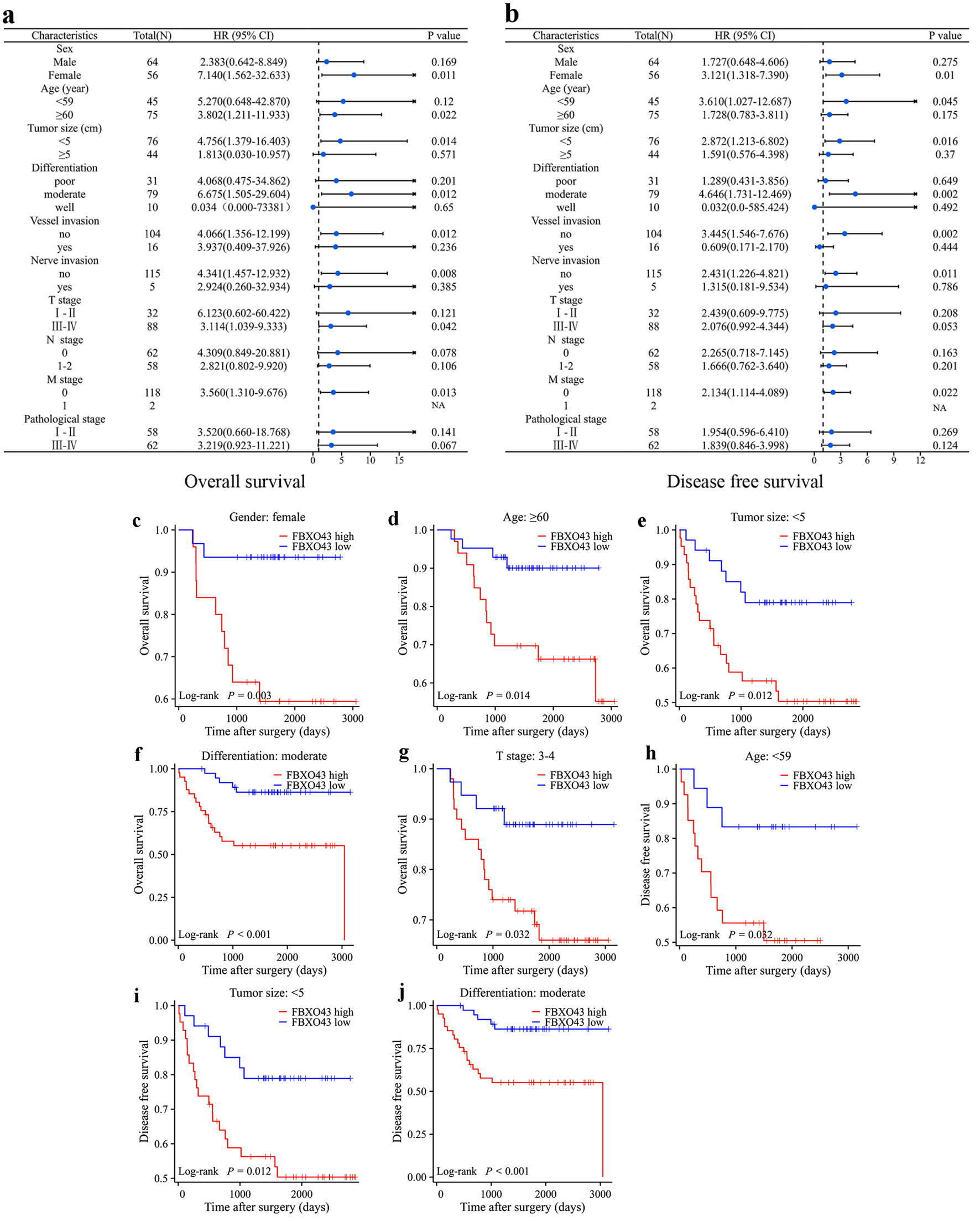 Click for large image | Figure 3. Prognostic value of FBXO43 for subgroup stratification of CRC. (a, b) Subgroup analysis of OS (a) and (b) by Cox regression according to the FBXO43 levels in cohort 1. (c) The OS of patients (female) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (d) The OS of patients (age ≥ 60) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (e) The OS of patients (tumor size < 5 cm) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (f) The OS of patients (moderate differentiation) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (g) The OS of patients (T stage III - IV) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (h) The DFS of patients (age < 59) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (i) The DFS of patients (tumor size < 5 cm) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. (j) The DFS of patients (moderate differentiation) in cohort 1 was compared between FBXO43 high and FBXO43 low groups. FBXO43: F-box protein 43; CRC: colorectal cancer; OS: overall survival; DFS: disease-free survival; HR: hazard ratio; CI: confidence interval. |
FBXO43 was associated with chemotherapy resistance in CRC patients
To explore the potential biological function of FBXO43 in CRC, differential expression profiles were examined between TCGA CRC samples that expressed high (70-100%) and low (0-30%) levels of FBXO43. A total of 84 upregulated genes and 113 downregulated genes were identified in the group with high expression of FBXO43 (|log2FC| > 1, adjusted P < 0.05) (Fig. 4a). The results of the KEGG signaling pathway enrichment analysis indicate that the DEGs were significantly enriched in various metabolic pathways, including drug metabolism - cytochrome P450, complement and coagulation cascades, metabolism of xenobiotics by cytochrome P450, tyrosine metabolism, retinol metabolism, and linoleic acid metabolism (Fig. 4b). Besides, GSEA was ulteriorly conducted in patients with high and low expression of FBXO43 in TCGA, and several drug-metabolism related pathways were conspicuously restrained including drug induction of bile acid pathway, drug metabolism other enzymes, drug metabolism cytochrome P450 and drug absorption, distribution, metabolism, and excretion (ADME) (Fig. 4c). Hence, we postulated that FBXO43 may contribute to chemotherapy response for CRC patients. Subsequent analysis of data from 94 out of 120 patients (43 out of 60 in FBXO43 low group and 51 out of 60 in FBXO43 high group), who underwent surgical resection and received at least three courses of adjuvant folinic acid/5-fluorouracil/oxaliplatin (FOLFOX) chemotherapy was conducted to validate the efficacy of FBXO43 in adjuvant chemotherapy. According to the results, patients with high FBXO43 levels had significantly shorter OS and DFS than those with low levels (Fig. 4d, e). Among patients undergoing adjuvant chemotherapy, those who exhibited recurrence of tumor within 1 year were considered chemotherapy-resistant, and those with a DFS more than 5 years were considered chemotherapy-sensitive. We further reviewed the expression of FBXO43 in primary CRC tissues obtained from chemotherapy-resistant patients. There is a significant increase of FBXO43 expression in chemotherapy-resistant patients’ primary CRC tissues compared to those with chemotherapy-sensitive CRC (Fig. 4f). These results suggest that patients with elevated levels of FBXO43 expression may not derive therapeutic benefits from chemotherapy following surgical resection.
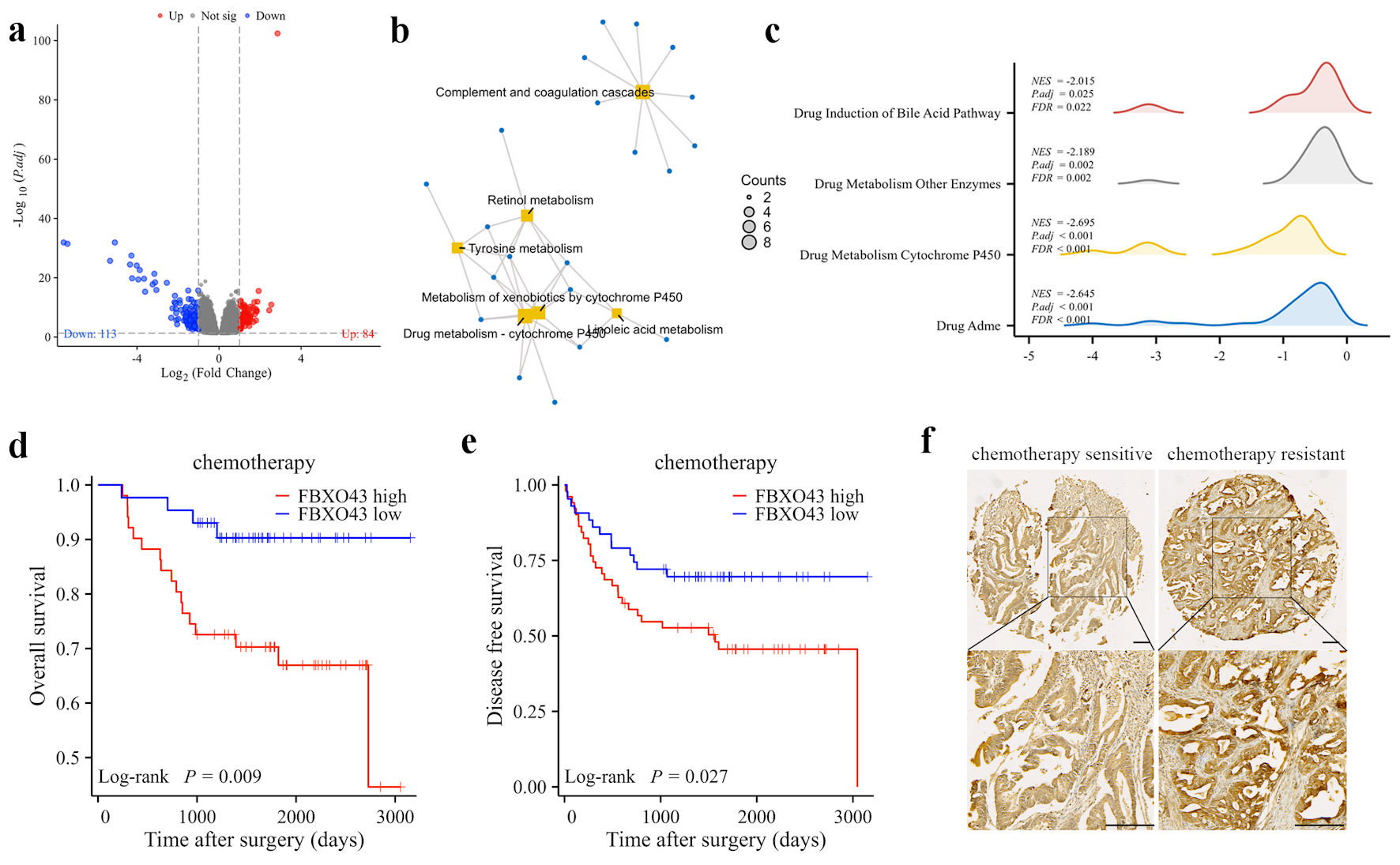 Click for large image | Figure 4. FBXO43 was associated with chemotherapy resistance in CRC patients. (a) Differential expression genes (DEGs) between FBXO43 high and FBXO43 low groups in the TCGA cohort were shown by volcano plots. Each red dot indicates an upregulated gene, and each blue dot indicates a downregulated gene (fold change > 1, adjusted P < 0.05). (b) Results of KEGG pathway analysis of DEGs (fold change > 1, adjusted P < 0.05). (c) Results of GSEA enrichment analysis of DEGs. (d, e) The OS and DFS of patients who receiving chemotherapy in cohort 1 after surgery resection were compared between FBXO43 high and FBXO43 low groups. (f) Representative IHC staining of FBXO43 expression in chemotherapy-resistance and chemotherapy-sensitive CRC tissues (scale bar: 100 µm). FBXO43: F-box protein 43; CRC: colorectal cancer; OS: overall survival; DFS: disease-free survival; HR: hazard ratio; TCGA: The Cancer Genome Atlas; IHC: immunohistochemistry; KEGG: Kyoto Encyclopedia of Genes and Genomes; GSEA: Gene Set Enrichment Analysis. |
| Discussion | ▴Top |
The present study reveals that FBXO43 was significantly upregulated in CRC, as evidenced by the analysis of the TCGA database. This finding was subsequently confirmed by qPCR and IHC, which demonstrated an upregulation of FBXO43 mRNA and protein levels in CRC tissues. Additionally, a majority of the 120 CRC patients (56.67%) exhibited higher tumoral expression of FBXO43 compared to para-tumor tissues (Fig. 1). Moreover, the expression of FBXO43 was found to be significantly increased in CRC patients with advanced T stage (III - VI), poorly differentiated tumors, and lymphatic metastasis. Previous studies have demonstrated the crucial involvement of FBXO43 in tumorigenesis, with upregulation observed in various malignancies such as HCC [14, 15], breast cancer [10], and cholangiocarcinoma [11]. The activation of the PI3K/AKT/P27 signaling pathway via FBXO43 has been found to promote the progression of cholangiocarcinoma [11]. Conversely, the inhibition of FBXO43 has been demonstrated to significantly impede the malignant progression of breast cancer cells by means of the degradation of proliferating cell nuclear antigen (PCNA) [10]. Taken together, these findings suggest that FBXO43 may represent a commonly differentially expressed gene in various types of cancer.
Results of univariate and multivariate analyses, along with Kaplan-Meier analysis, indicate that FBXO43 is an autonomous risk factor for OS in CRC patients (Fig. 2), which highlights the clinical prognostic potential of FBXO43. Additionally, male gender and advanced age are widely acknowledged as risk factors for CRC. Furthermore, larger tumor size, advanced pathologic stage, and invasion of vessel or nerve are commonly linked to poorer survival outcomes [1, 16, 17]. Prognostic value of FBXO43 for OS and DFS was further identified in these subgroups regarding age, sex, tumor size, pathological stage, and invasion (Fig. 3). The findings of our study indicate that a heightened expression of FBXO43 can differentiate cohorts with inferior survival rates, particularly in individuals with lower risk profiles (i.e., female gender, age below 60 years, tumor size less than 5 cm, absence of nerve or vessel invasion, and no metastasis). Based on these results, FBXO43 may serve as a valuable prognostic marker and complement other risk factors in forecasting survival outcomes.
To explore the function of FBXO43, KEGG and GSEA functional enrichment analysis were performed using TCGA data, which showed that CRC patients with high level of FBXO43 exhibited significant inhibition of both the hsa00980 (metabolism of xenobiotics by cytochrome P450) and hsa00982 (drug metabolism) pathways. Cytochrome p450 enzymes (CYPs) is the main enzymes involved in drug metabolism, and it has been found that the combination of pathway hsa00980 (metabolism of xenobiotics by cytochrome P450) and hsa00982 (drug metabolism) contributes to drug resistance or adverse reaction during chemotherapy for gastric adenocarcinoma [18]. We further suspected that patients with high level of FBXO43 may be resistant to chemotherapy due to defects in drug metabolism. All stage III and a portion of stage II or stage IV CRC patients were recommended to receive adjuvant chemotherapy, and 5-fluorouracil (5-FU) based chemotherapy is the most common treatment. To explore the role of FBXO43 in CRC chemotherapy, 94 CRC patients treated with at least three courses of standard FOLFOX chemotherapy regimens after surgery were retrospectively analyzed in our study. Survival analysis indicated that patients with low expression of FBXO43 showed a significantly preferable prognosis, suggesting that these patients may benefit from chemotherapy while those with high expression of FBXO43 may not. In addition, we observed that FBXO43 was distinctly elevated in chemotherapy-resistant patients’ primary CRC tissues before receiving chemotherapy. These results suggest that FBXO43 may be a potential biomarker in selection of CRC patients for chemotherapy.
Although the relationship between FBXO43 and chemotherapy has not been reported, in our knowledge, other researchers have reported its relationship with chemo-resistant genes. For example, p53 is widely accepted as a responsible gene for chemotherapy resistance [19, 20]. Zhou et al raised that methyltransferase 3 (METTL3)/insulin like growth factor 2 (IGF2BP2)-mediated upregulation of FBXO43 promotes HCC malignancy by stimulating p53 degradation in a UBE2C-dependent manner [9]. Similarly, FBXO43 has been reported to facilitate cancer progression by increasing stability of CCND1 [14]; and in vitro experiments showed that inhibition of CCND1 reduces the 5-FU resistance in CRC cells [21]. Collectively, the above data suggested a FBXO43 mediated chemotherapy resistance mechanism, however, further research is required to determine how FBXO43 disrupts these metabolic pathways and results in chemotherapy resistance.
Nevertheless, there are several things to mention. Firstly, our study collected data and samples in a retrospective manner, and further investigation involving a prospective validation cohort is worthwhile. Secondly, due to the small number of cases in some subgroups in our cohort, such as stage M1, further research is needed on FBXO43’s role in these subgroups. Thirdly, the biological function of FBXO43 in CRC is not well known, which needs in vivo and in vitro experiments for further exploration.
In summary, we found that upregulated expression of FBXO43 in tumor tissues was associated with poor OS and DFS for CRC patients. Moreover, patients with high FBXO43 may not benefit from chemotherapy after surgical resection. Our data suggested that FBXO43 may serve as a biomarker for prognostic and chemotherapeutic prediction.
| Supplementary Material | ▴Top |
Suppl 1. Clinicopathologic features of patients in cohort 1.
Suppl 2. Univariate and multivariate analysis of overall survival in cohort 1.
Suppl 3. Univariate and multivariate analysis of disease-free survival in cohort 1.
Suppl 4. Kaplan-Meier plot of OS and DSS between the “high FBXO43” and “low FBXO43” CRC patients of TCGA.
Acknowledgments
None to declare.
Financial Disclosure
This work was sponsored by Natural Science Foundation of Xinjiang Uygur Autonomous Region (2022D01C344) and "Karakoram" Talent Fund of General Hospital of Xinjiang Military Command (2022JC001).
Conflict of Interest
No potential conflicts of interest were disclosed.
Informed Consent
Informed consent was obtained from all participants.
Author Contributions
Acquisition of data: Junyu Liu, Miao Li, Xi Yang, Yingying Liu and Yulan Wang; analysis and interpretation of data: Junyu Liu, Miao Li and Xi Yang; drafting of the manuscript: Junyu Liu, Xi Yang and Shichao Li; financial support: Shichao Li; critical revision of the manuscript: Shichao Li and Fengping Zheng; Study concept and design: Junyu Liu and Fengping Zheng.
Data Availability
All data that support the findings of this study are available to the corresponding authors by reasonable request.
Abbreviations
CRC: colorectal cancer; 5-FU: 5-fluorouracil; FBXO43: F-box protein 43; CYPs: cytochrome p450 enzymes; EMI2: endogenous meiotic inhibitor 2; OS: overall survival; TCGA: The Cancer Genome Atlas; DFS: disease-free survival; KEGG: Kyoto Encyclopedia of Genes and Genomes; TNM: tumor-nodes-metastasis; ssGSEA: single sample Gene Set Enrichment Analysis; GSEA: Gene Set Enrichment Analysis; DEGs: differentially expressed genes; TMA: tissue microarray; METTL3: methyltransferase 3; IGF2BP2: insulin like growth factor 2; UBE2C: ubiquitin conjugating enzyme E2 C; PCNA: proliferating cell nuclear antigen
| References | ▴Top |
- Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, van de Velde CJ, et al. Colorectal cancer. Nat Rev Dis Primers. 2015;1:15065.
doi pubmed pmc - Strum WB. Colorectal adenomas. N Engl J Med. 2016;374(11):1065-1075.
doi pubmed - Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, Cercek A, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin. 2020;70(3):145-164.
doi pubmed - Ladabaum U, Dominitz JA, Kahi C, Schoen RE. Strategies for colorectal cancer screening. Gastroenterology. 2020;158(2):418-432.
doi pubmed - U. S. Preventive Services Task Force, Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, et al. Screening for colorectal cancer: US preventive services task force recommendation statement. JAMA. 2021;325(19):1965-1977.
doi pubmed - Shoji S, Muto Y, Ikeda M, He F, Tsuda K, Ohsawa N, Akasaka R, et al. The zinc-binding region (ZBR) fragment of Emi2 can inhibit APC/C by targeting its association with the coactivator Cdc20 and UBE2C-mediated ubiquitylation. FEBS Open Bio. 2014;4:689-703.
doi pubmed pmc - Xu B, Lv W, Li X, Zhang L, Lin J. Prognostic genes of hepatocellular carcinoma based on gene coexpression network analysis. J Cell Biochem. 2019;120(7):11616-11623.
doi pubmed - Wu S, Qin L, Yang J, Wang J, Shen Y. Association between F-box-only protein 43 overexpression and hepatocellular carcinoma pathogenesis and prognosis. Cancer Med. 2023;12(8):10062-10076.
doi pubmed pmc - Zhou H, Zeng C, Liu J, Luo H, Huang W. F-Box Protein 43, Stabilized by N6-methyladenosine methylation, enhances hepatocellular carcinoma cell growth and invasion via promoting p53 degradation in a ubiquitin conjugating enzyme E2 C-dependent manner. Cancers (Basel). 2023;15(3):957.
doi pubmed pmc - Ma R, Zhu K, Yuan D, Gong M, Li Y, Li K, Meng L. Downregulation of the FBXO43 gene inhibits tumor growth in human breast cancer by limiting its interaction with PCNA. J Transl Med. 2021;19(1):425.
doi pubmed pmc - Zhou S, Qu KL, Li JA, Chen SL, Zhang YG, Zhu C, Jin H, et al. YY1 activates EMI2 and promotes the progression of cholangiocarcinoma through the PI3K/Akt signaling axis. Cancer Cell Int. 2021;21(1):699.
doi pubmed pmc - Sun W, Li SC, Xu L, Zhong W, Wang ZG, Pan CZ, Li J, et al. High FLT3 levels may predict sorafenib benefit in hepatocellular carcinoma. Clin Cancer Res. 2020;26(16):4302-4312.
doi pubmed - https://portal.gdc.cancer.gov/
- Li CM, Zhang J, Wu W, Zhu Z, Li F, Wu D, Wang XJ, et al. FBXO43 increases CCND1 stability to promote hepatocellular carcinoma cell proliferation and migration. Front Oncol. 2023;13:1138348.
doi pubmed pmc - Ma R, Liu W, Sun T, Dang C, Li K. Clinical significance of FBXO43 in hepatocellular carcinoma and its impact on tumor cell proliferation, migration and invasion. PeerJ. 2023;11:e15373.
doi pubmed pmc - Kanth P, Inadomi JM. Screening and prevention of colorectal cancer. BMJ. 2021;374:n1855.
doi pubmed - Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet. 2019;394(10207):1467-1480.
doi pubmed - Zhao L, Lei H, Shen L, Tang J, Wang Z, Bai W, Zhang F, et al. Prognosis genes in gastric adenocarcinoma identified by cross talk genes in disease-related pathways. Mol Med Rep. 2017;16(2):1232-1240.
doi pubmed pmc - Van der Jeught K, Xu HC, Li YJ, Lu XB, Ji G. Drug resistance and new therapies in colorectal cancer. World J Gastroenterol. 2018;24(34):3834-3848.
doi pubmed pmc - Huang Y, Liu N, Liu J, Liu Y, Zhang C, Long S, Luo G, et al. Mutant p53 drives cancer chemotherapy resistance due to loss of function on activating transcription of PUMA. Cell Cycle. 2019;18(24):3442-3455.
doi pubmed pmc - Huang R, Lin JY, Chi YJ. MiR-519d reduces the 5-fluorouracil resistance in colorectal cancer cells by down-regulating the expression of CCND1. Eur Rev Med Pharmacol Sci. 2018;22(9):2869-2875.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


