| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 15, Number 3, June 2024, pages 432-453
Epidemiology of Adenosquamous Carcinomas
Matthew G.K. Benescha, g, h , Vicente O. Ramos-Santillana, g, Colin J. Roga, Erek D. Nelsona, Kazuaki Takabea, b, c, d, e, f, h
aDepartment of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
bDepartment of Breast Surgery and Oncology, Tokyo Medical University, Tokyo 160-8402, Japan
cDepartment of Gastroenterological Surgery, Yokohama City University Graduate School of Medicine, Yokohama 236-0004, Japan
dDivision of Digestive and General Surgery, Niigata University Graduate School of Medical and Dental Sciences, Niigata 951-8520, Japan
eDepartment of Breast Surgery, Fukushima Medical University School of Medicine, Fukushima 960-1295, Japan
fDepartment of Surgery, University at Buffalo Jacobs School of Medicine and Biomedical Sciences, State University of New York, Buffalo, NY 14263, USA
gThese authors equally contributed to this work.
hCorresponding Author: Matthew G.K. Benesch, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA; Kazuaki Takabe, Department of Surgical Oncology, Roswell Park Comprehensive Cancer Center, Buffalo, NY 14263, USA
Manuscript submitted March 7, 2024, accepted April 15, 2024, published online May 7, 2024
Short title: Epidemiology of Adenosquamous Carcinomas
doi: https://doi.org/10.14740/wjon1845
| Abstract | ▴Top |
Background: Adenosquamous carcinomas (ASCs) are a very rare histology containing cancer cells with both glandular-like (adeno) and squamous cell histologies, comprising typically a fraction of a percent of all solid tumors. The bulk of the literature on ASCs is comprised of case reports and small series, with the general finding that ASCs tend to have worse outcomes than either of their parent histologies. However, there is a lack of pan site-comparative studies in the literature that compare ASC clinicodemographic and survival outcomes with those of conventional adenocarcinomas (ACs) and squamous cell carcinomas (SCCs).
Methods: In this study, we summarize these outcomes in eight primary sites, comprising 92.7% of all ASC cases diagnosed from 1975 to 2020 in the Surveillance, Epidemiology, and End Results (SEER) database.
Results: Lung ASCs comprise 51.5% of all ASC cases, accounting for 1.1% of all lung cancer cases, followed by uterine/cervical cancers at 29.7% of all ASC cases, translating into 1.8% of all cancers in this site. In descending order, the remaining 20% of ASCs arise in pancreatic, oral cavity, biliary, esophageal, colorectal, and gastric sites, comprising between 0.1% and 0.7% of all cancers in these sites. Apart from pancreatic and oral cavity cancers, ASC tumors tended to favor higher rates of regional or distant disease at presentation with poor tumor differentiation compared to either AC or SCC histologies. After multivariable analysis, adjusting for age, sex, detection stage, grade differentiation, surgery, chemotherapy, and radiotherapy, except for oral cavity cancers, ASCs tended to have worse overall survivals compared to ACs (hazard ratios: 1.1 - 1.6) and SCC (1.0 - 1.3), with colorectal ASCs having the worse overall survival compared to colorectal ACs, with a hazard ratio of 1.4 (95% confidence interval: 1.3 - 1.6).
Conclusions: Overall, these results suggest that ASC outcomes are site specific, and in general, tend to have worse outcomes than nonvariant ACs and SCCs even after correction for common clinical and epidemiological factors. These cancers have a poorly understood but unique tumor biology that warrants further characterization.
Keywords: Cancer; Chemotherapy; Demographics; Histopathology; Outcomes; Radiotherapy; Surgery
| Introduction | ▴Top |
Adenosquamous carcinomas (ASCs) are a rare histological class of cancer that contain both squamous cells and glandular-like (adeno) cells [1, 2]. In certain cancers, this histology has been associated with more aggressive disease than either parent histology [3]. These cancers are recognized by the International Classification of Diseases for Oncology (ICD)-0-3 classification as a unique entry (8560/3) under the broader umbrella of Complex Epithelial Neoplasms [4]. The World Health Organization varies the formal definition of ASCs slightly based on tumor site, where for lungs, each of the squamous and adenocarcinoma components must constitute at least 10% of the tumor, and for gastrointestinal malignancies, the squamous component constitutes at least 25% of the tumor [1, 2].
Given that these cancers comprise less than 1% of all total carcinomas, most of the literature on ASCs is limited to case reports or small series [5, 6]. There are no studies that systematically and robustly compare ASCs to nonvariant histologies, which limits the ability of researchers and clinicians to make meaningful comparisons to conventional adenocarcinomas (ACs) and squamous cell carcinomas (SCCs) [7]. The systematic examination and characterization of the epidemiological and clinical behavior of this histology requires the resources only available through a population-level database [8].
The Surveillance, Epidemiology, and End Results (SEER) database is an amalgamation of population-based cancer registries maintained by the National Cancer Institute, encompassing 47.9% of the United States population with its most recent release [9]. As one of the oldest population-level cancer registries, it is a well-respected resource for investigating correlations between histopathological data with patient outcomes across all cancer sites with nearly 50 years of data capture [10]. In this site-stratified analysis, we use the SEER database to overcome the limitations imposed by cancer rarity to investigate the clinicopathological characteristics and survival outcomes of ASCs compared to nonvariant ACs and SCCs across all major locations. This investigation provides a thorough epidemiological description and characterization of this poorly understood rare cancer histology.
| Materials and Methods | ▴Top |
Patient selection
The National Cancer Institute’s SEER database composed from 18 SEER cancer registries was employed using data from 1975 to 2020, as previously described [11-17]. Data release from the SEER database does not require informed patient consent or review by an institutional review board. The SEER database was accessed and searched in compliance with signed user agreements [17]. A complete outline of exclusion criteria and its effects on case counts is presented here (Supplementary Material 1, www.wjon.org). Definitions of variables analyzed are presented here (Supplementary Material 2, www.wjon.org). We limited our analysis to sites with at least 400 cases of undifferentiated carcinomas, which captures 92.7% of all cases (Supplementary Material 3, www.wjon.org). We compared demographic and outcomes data for ASCs to site-matched ACs and SCCs. All cases listed in SEER have at least a tissue biopsy reviewed by a pathologist to confirm the histological diagnosis [17].
Statistical analysis
All selected data from 18 SEER cancer registries were imported into Stata 15.1 (StataCorp LLC, College Station, TX, USA) for statistical analysis following case listing downloading using SEER*Stat 8.4.2 (Surveillance Research Program, National Cancer Institute, Calverton, MD, USA). A complete case analysis was completed after variable definition, as previously described (Supplementary Material 1, 2, www.wjon.org) [11-17].
Baseline patient characteristics were compared with the t and Chi-squared tests for continuous and categorical variables, respectively. Univariate and multivariable Cox proportional hazard regression was used to determine the association of mortality with cancer histology type, adjusting for age, sex, race, detection stage, grade differentiation, surgery, chemotherapy, and radiotherapy. All hazard ratios (HRs) were calculated with 95% confidence intervals. Use of surgery, chemotherapy, and radiotherapy as treatment variables were binary. All P values were two-sided, with a threshold of 0.05 to determine statistical significance. Survival curves were plotted using the Kaplan-Meier method, with P values for survival curves generated by the log rank test. Graphs were plotted using Origin Pro 2022 (OriginLab Corporation, Northampton, MA, USA). Using SEER 18 (2000 - 2020) data with SEER*Stat 8.4.2, incidence rates were calculated and age-adjusted to the 2000 United States standard population with the age variable recode < 1-year-olds. Cause-specific survival and relative survival were calculated with the same dataset and software and were both age standardized to the International Cancer Survival Standard 1-Age 15+ variable via the actuarial method, and Ederer II cumulative expected method for relative survival.
| Results | ▴Top |
Analysis of ASCs by anatomical site
Subsections are presented in order of anatomical site by the percentage of ASC cases in that site relative to all cases of ASC presented (92.7% of all cases of ASC in the SEER database) (Fig. 1). Within each subsection, we first present a demographics table with all included cancer cases for that site, followed by nonvariant AC and SCC cases, and then the ASC cases. The second table presents both univariate and multivariable HRs for cause-specific mortality according to the same demographic, histopathological, and treatment variables as the first table, comparing ASCs to the other cancer categories.
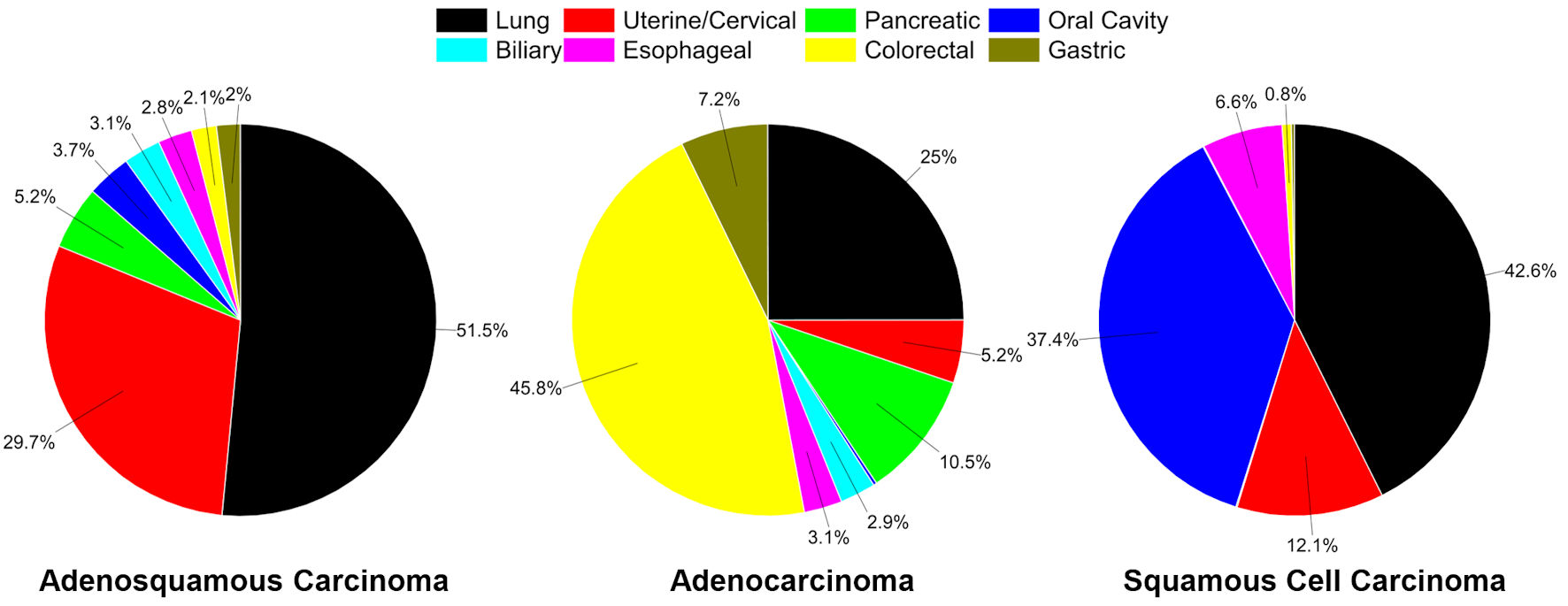 Click for large image | Figure 1. Distribution of cancers in SEER, 1975 - 2020 for sites analyzed. Distribution of adenosquamous carcinoma cases in all major sites examined (total of 26,525 cases (92.7% of all adenosquamous carcinoma cases in SEER)) (left). Distribution of all conventional adenocarcinoma cases in sites examined (total of 1,535,527 cases) (middle). Distribution of all conventional squamous cell carcinoma cases in sites examined (total of 588,802 cases) (right). |
To provide a visual overview across all cancer sites, Kaplan-Meier survival curves with 95% confidences intervals are also presented (Fig. 2).
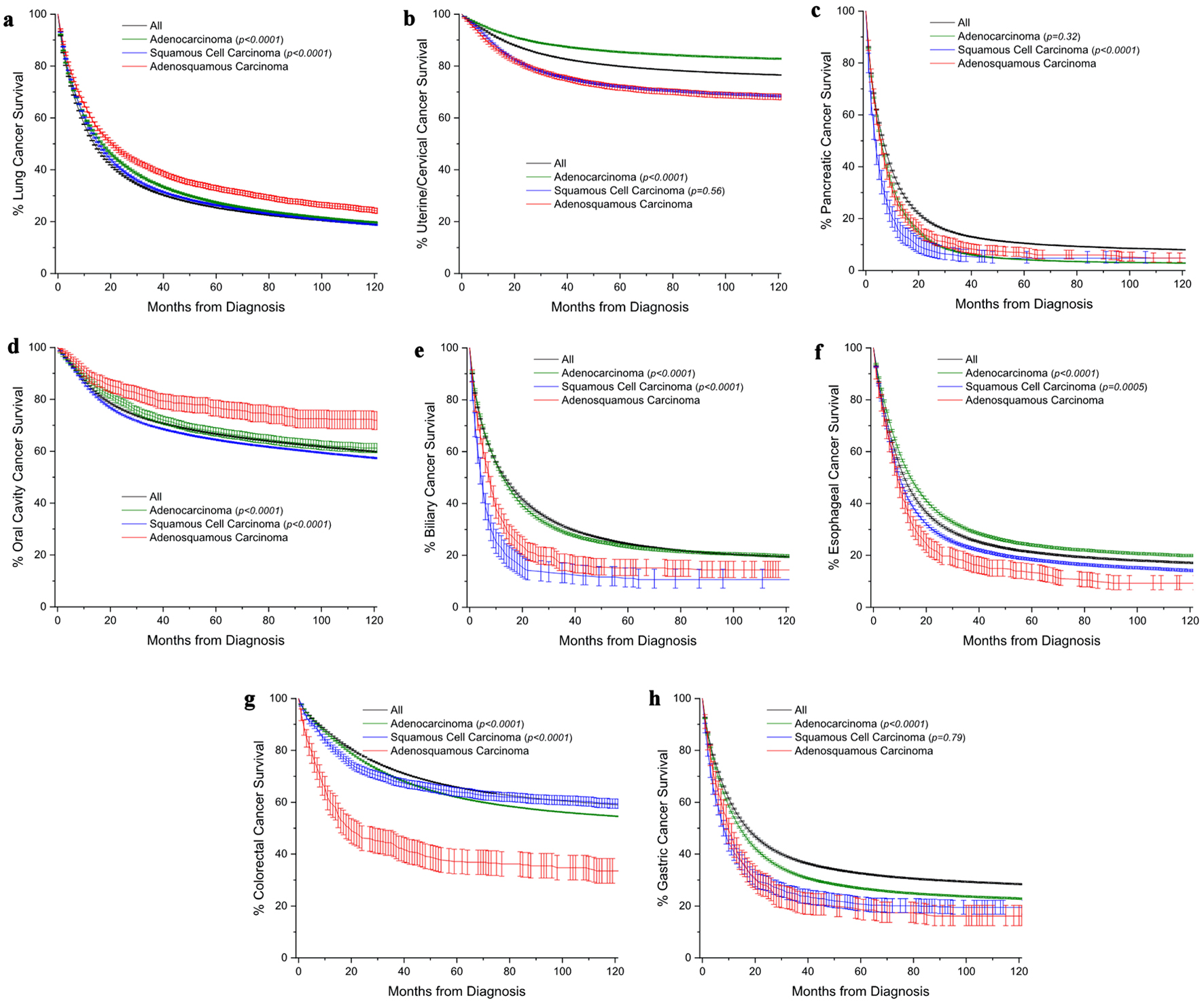 Click for large image | Figure 2. Kaplan-Meier survival curves comparing overall survival of adenosquamous carcinomas patients to those with either conventional adenocarcinomas or squamous cell carcinomas. The black line represents a comparative survival curve for all malignancies within that site. (a) Lung cancer. (b) Uterine/cervical cancer. (c) Pancreatic cancer. (d) Oral cavity cancer. (e) Biliary cancer. (f) Esophageal cancer. (g) Colorectal cancer. (h) Gastric cancer. All survival curves are shown with 95% confidence interval. Among subtypes, pairwise statistical comparisons by the log-rank test are shown relative to adenosquamous carcinomas. |
Lung cancer
ASC comprised a minority of lung cancer cases at 1.1% but represented just over half (51.5%) of all ASC tumors (Table 1, Fig. 1). Age of diagnosis was overall similar among all cancer subtypes in the 68 - 70 years old range (Table 1). Compared to males, female cases were slightly lower for ASC (44.3%) and SCC (35.9%), but the same for AC (50%). ASC tended to be diagnosed at an earlier stage (26.1%), in contrast to AC (19.9%) but very similar to SCC (24.5%). Notably, ASC tumors were more frequently poorly differentiated (44.9%) compared to AC (26.1%) and SCC (32.8%). About half of the ASC patients underwent surgical resection, contrasted with about a quarter for both AC and SCC. Furthermore, patients with AC and SCC underwent chemotherapy (41.0% and 35.4%, respectively) and radiotherapy more frequently (39.4% and 46.7%, respectively) than patients with ASC (34.3% for chemotherapy and 53.9% for radiotherapy). Survival was significantly better at all points for ASC patients. Median survival was 16.6 months compared to 13.7 months for AC and 13.5 months for SCC (Table 1). In comparison to AC and SCC lung cancers, ASC had an unadjusted HR of 0.87 (95% confidence interval 0.85 - 0.89) to AC and 0.84 (0.82 - 0.86) to SCC, but after multivariable analysis correcting for age, sex, race, detection stage, grade, and use of surgery, chemotherapy, and radiotherapy, the HRs were 1.14 (1.11 - 1.16), and 1.08 (1.05 - 1.10), respectively (Table 2).
 Click to view | Table 1. Demographics and Clinical Characteristics for Lung Cancer by Histology |
 Click to view | Table 2. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Lung Cancer |
Uterine/cervical cancer
ASC represented 1.8% of uterine/cervical cancer cases and 29.7% of all ASC cases (Table 3, Fig. 1). The mean age at presentation for ASC was 55.7 years old, which was 4.7 years older compared to SCC but 5.1 years younger than AC (Table 3). ASC tended to be detected at a localized stage more frequently than SCC (52.1% vs. 46.5%, respectively), but less often when compared to AC (74.6%). Further, ASC presented with distant disease more frequently (14.9%) than AC and SCC (7.7% and 11.3%, respectively). Notably, ASC tumors were more frequently poorly differentiated (44.0%) compared to AC (17.3%) and SCC (28.6%). Seventy-eight percent of ASC patients underwent surgical resection, contrasting with 85.6% for AC and 54.2% for SCC. Patients with ASC and SCC underwent chemotherapy (26.0% and 37.0%, respectively) and radiotherapy more frequently (56.0% and 57.1%, respectively) compared to individuals diagnosed with AC, where the rates were lower (9.1% for chemotherapy and 32.2% for radiotherapy). ASC survival rates closely matched SCC rates, which at 10 years was significantly worse compared to AC patients (about 66% vs. about 78%, respectively) (Table 3). In comparison to AC and SCC uterine/cervical cancers, ASC had a HR of 1.06 (1.01 - 1.11) and 1.18 (1.13 - 1.23), respectively, after multivariate analyses (Table 4).
 Click to view | Table 3. Demographics and Clinical Characteristics for Uterine/Cervical Cancer by Histology |
 Click to view | Table 4. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Uterine/Cervical Cancer |
Pancreatic cancer
ASC histology constituted 0.5% of all pancreatic cancers and 5.2% of all ASCs (Table 5, Fig. 1). Age of presentation was similar among ASC, AC, or SCC (about 68 years old), as well as sex distribution (about 52% males) (Table 5). ASC tumors were less likely to present as distant disease (49.6%) than either AC or SCC (57.9% vs. 62.1%, respectively). However, these tumors presented with higher rates of poor grade differentiation than either AC or SCC (33.7%, vs. 15.0% and 30.8%, respectively). ASC patients had significantly higher surgery rates at 36.2% compared to AC at 13.3% and SCC at 8.3%. Median survival for ASC and AC patients was similar at just under 6 months, compared to about 4 months for SCC patients (Table 5). In comparison to AC, ASC had an equivalent unadjusted HR of 0.97 (0.91 - 1.03) to AC and 0.77 (0.68 - 0.86) to SCC, but after multivariable analysis, the HRs were 1.25 (1.18 - 1.34), and 1.16 (1.02 - 1.31), respectively (Table 6).
 Click to view | Table 5. Demographics and Clinical Characteristics for Pancreatic Cancer by Histology |
 Click to view | Table 6. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Pancreatic Cancer |
Oral cavity cancer
As a collective group, ASCs comprised 0.4% of all oral cavity cancers and 3.7% of all ASCs (Table 7, Fig. 1). These cancers had about the same average age of onset at about 63 - 64 years across all three histologies (Table 7). ASC tumors were similar to AC tumors with a male prevalence in the mid-50% range, whereas for SCC tumors, 72.6% of cases were in male patients. ASC tumors were most often detected as localized disease 53.2% of the time, compared to 38.8% and 30.6% of AC and SCC tumors, respectively. AC tumors had higher rates of poor differentiation at 34.7% than either SCC or ASC tumors (23.9% vs. 25.3%, respectively), though ASC tumors had a very high rate of unknown grade differentiation at 43.9%. ASC tumors were treated with surgery 84.0% of the time, similar to AC at 78.0%, vs. 55.4% of time for the SCC, while SCC tumors were more likely treated with chemotherapy (35.5% vs. about 14% for AC and ASC), with similar rates of radiotherapy in the 50-60% range for all histologies. Ten-year survival rates were about 15% higher for ASC tumors than either AC or SCC tumors (Table 7). This translated into overall better HR for mortality for ASC vs. AC, though not statistically significant after multivariable adjustment at 0.88 (0.76 - 1.02), and for ASC vs. SCC 0.75 (0.66 - 0.86) (Table 8).
 Click to view | Table 7. Demographics and Clinical Characteristics for Oral Cavity Cancer by Histology |
 Click to view | Table 8. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Oral Cavity Cancer |
Biliary tract cancer
As a collective group, ASCs comprised 0.3% of all biliary tract cancers and 3.1% of all ASCs (Table 9, Fig. 1). These cancers had about the same average age of onset at about 68 - 69 years old across all three histologies (Table 9). ASC tumors were more similar to SCC tumors with a female rate at about 65%, whereas for AC tumors, 56.2% of cases were in female patients. ASC tumors were most often detected as regional disease 44.7% of the time, compared to 39.4% and 34.2% of AC and SCC tumors, respectively, with higher rates of poor differentiation at 43.8% compared to 36.9% and 39.8%. ASC tumors were treated with surgery 76.9% of the time, which was much higher than AC at 51.3% or SCC at 42.4%. Chemotherapy and radiotherapy use rates were statistically identical across all histologies. Median survival for ASC patients was about 8 months, compared to about 11 months for AC patients and 4 months for SCC patients (Table 9). This translated into overall worse morality HR for ASC compared to AC at 1.56 (1.43 - 1.71), but equivalent to SCC 0.93 (0.80 - 1.09) (Table 10).
 Click to view | Table 9. Demographics and Clinical Characteristics for Biliary Tract Cancer by Histology |
 Click to view | Table 10. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Biliary Tract Cancer |
Esophageal cancer
ASC histology constituted 0.7% of all esophageal cancers and 2.8% of all ASCs (Table 11, Fig. 1). The age of presentation was similar among ASC, AC, and SCC (about 66 - 67 years old). Sex distribution towards males was similar for ASC and AC at 82.3% and 86.1% respectively, but lower for SCC at 65.1% (Table 11). ASC tumors were most likely to present as distant disease (41.4%), compared to 38.0% for AC and 28.3% for SCC. Additionally, ASC tumors presented with higher rates of poor grade differentiation than either AC or SCC (60.0%, vs. 37.3% and 33.8%, respectively). ASC patients had similar surgery rates at 33.1% compared to AC at 32.9%. SCC tumors had higher rates of radiotherapy use at 63.2%, compared to about 55% for the other two histologies. Median survival for ASC and SCC patients was similar at 8 - 10 months, compared to about 14 months for AC patients (Table 11). After multivariable analysis, ASC patients had worse mortality HR at 1.30 (1.19 - 1.42) compared to AC patients, and similarly to SCC patients at 1.21 (1.10 - 1.32) (Table 12).
 Click to view | Table 11. Demographics and Clinical Characteristics for Esophageal Cancer by Histology |
 Click to view | Table 12. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Esophageal Cancer |
Colorectal cancer
ASC histology constituted 0.1% of all colorectal cancers and 2.1% of all ASCs (Table 13, Fig. 1). ASC patients had a mean age of presentation of 64.6 years, while AC patients were older at 67.5 years, and SCC patients were younger at 62.9 years (Table 13). There was a female bias at 52.1% for ASC, increased to 66.7% for SCC, but decreased to 47.4% for AC tumors. ASC tumors were most likely to present as distant disease (42.3%), compared to 22.3% for AC and 17.8% for SCC. Additionally, ASC tumors presented with much higher rates of poor grade differentiation than either AC or SCC (61.5%, vs. 17.1% and 32.0%, respectively). Therapeutic modality use for ASC patients was similar to AC patients, apart from increased chemotherapy for ASC patients (46.5% vs. 36.5%). Median survival for ASC patients was about 17 - 20 months, while median survival was not reached in either the AC or SCC groups (Table 13). After multivariable analysis, ASC patients had worse mortality HR at 1.44 (1.28 - 1.63) compared to AC patients, and similarly to SCC patients at 1.26 (1.08 - 1.47) (Table 14).
 Click to view | Table 13. Demographics and Clinical Characteristics for Colorectal Cancer by Histology |
 Click to view | Table 14. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Colorectal Cancer |
Gastric cancer
ASC histology constituted 0.3% of all gastric cancers and 2.0% of all ASCs (Table 15, Fig. 1). ASC patients had an earlier mean age of presentation of 65.6 years old, compared to 68.5 years old for AC patients and 67.3 years old for SCC patients (Table 15). There was a strong male bias at 72.2% for ASC, compared to 66.5% for AC and 70.9% for SCC tumors. ASC tumors were most likely to present as distant disease at 43.0%, compared to 37.6% for AC and 41.4% for SCC. Similarly, ASC tumors presented with much higher rates of poor grade differentiation than either AC or SCC (63.9%, vs. 47.8% and 41.5%, respectively). Therapeutic modality use for ASC patients was similar to AC patients (surgery 54.9% vs. 48.6%, respectively), compared to 26.2% for SCC patients. ASC patients had the highest rates of chemotherapy use at 50.9%, compared to 41.0% for AC and 44.0% for SCC. Radiotherapy use was similar for ASC and SCC patients (33.4% vs. 38.7%). Median survival for ASC patients was about 9 - 10 months, compared to 14 - 17 months for AC patients and 7 - 8 months for SCC patients (Table 15). After multivariable analysis, ASC patients had worse mortality HR at 1.24 (1.11 - 1.38) compared to AC patients, but equivalent to SCC patients at 1.11 (0.96 - 1.27) (Table 16).
 Click to view | Table 15. Demographics and Clinical Characteristics for Gastric Cancer by Histology |
 Click to view | Table 16. Univariate and Multivariable Cox-Proportional Hazard Ratios (HRs) of Mortality for Gastric Cancer |
| Discussion | ▴Top |
This study is the most comprehensive comparative analysis of ASCs to site-matching parent nonvariant ACs and SCC histologies. Because these cancers comprise less than 0.3% of all malignancies, the literature on this histology is extremely spare. There is a pressing need to better understand the underlying tumor biology, as several reports have suggested that the incidence of these malignancies is rising [18, 19]. Gathered primarily from limited series, the prevailing understanding of ASC clinical behavior is that patients with these malignancies have more aggressive disease progression compared to site-matched ACs or SCCs [20, 21]. However, outside of a systematic examination of a population-level registry, the epidemiological factors that may be contributing to this perception cannot be well characterized.
From this analysis, we demonstrate site-specific variabilities in the behaviors of ASCs. Among the gastrointestinal tract malignancies, esophageal, gastric, and biliary tumors have slightly earlier ages of onset than either parent histology (about 1 - 3 years), identical for pancreatic cancer, and averaged between the histologies for colorectal cancer (median age AC 69 years old, SCC 63 years old, ASC 66 years old). With respect to stage at detection, ASCs of the lung, pancreas, oral cavity, and biliary tract tended to present with less distant disease, whereas uterine/cervical, esophageal, colorectal, and gastric cancers presented more often with distant disease than ACs or SCCs. The notable outlier was colorectal cancers, where ASCs presented with distant disease 42.3% of the time, nearly double that of the other histologies (AC 22.3%, SCC 17.8%). Except for oral cavity cancers, the grade differentiation was poor, nearly twice as often for ASCs at all other sites compared to ACs and SCCs. Taken all together, the unadjusted mortality HRs for ASCs compared to ACs were higher for uterine/cervical, biliary tract, esophageal, colorectal, and gastric cancers (about 1.3 - 2.2), equivalent for pancreatic cancer, and lower for lung and oral cavity cancers (about 0.7 - 0.9). Compared to SCCs, unadjusted mortality HRs were higher for esophageal and colorectal cancers (about 1.2 - 2.2), equivalent for uterine/cervical and gastric cancers, and lower for lung, pancreatic, oral cavity, and biliary tract cancers (about 0.7 - 0.8). However, after multivariable adjustment for sex, race, detection stage, grade differentiation, surgery, chemotherapy, and radiotherapy, except for oral cavity cancers, ASCs in all other sites had higher mortality HRs than ACs or SCCs (apart from biliary tract and gastric SCCs, which were equivalent). This finding implies that apart from oral cavity cancers, ASC histology may be an independent predictor of poorer prognosis compared to conventional ACs and SCCs.
The etiology of ASCs is poorly understood. Leading ideas include a predominant adenocarcinoma histology that undergoes a subsequent metaplasia, or in other cases, an underlying squamous metaplasia that experiences a malignant transformation secondary to persistent chronic inflammation [22-26]. Alternatively, these cancers may arise from pluripotent cancer stems cells capable of inducing malignant transformation of both histologies or transdifferentiation [6]. This theory is particularly intriguing, because if these cancers are more likely to be propagated by semi-quiescent cancer stem cells, which are well recognized to be chemotherapy and radiotherapy resistant, it might explain the higher rates of dedifferentiation and worsened outcomes compared to conventional histologies [27, 28].
This study does have several limitations. Despite this project using a well-validated population level database with quality assurance metrics [10], the research is retrospective in nature, and therefore is prone to selection bias. In order to obtain sufficient numbers of ASC cases for meaningful descriptive and comparative analyses across all major disease sites, the entire span of SEER database is required, which requires us to limit our treatment variables to binary values. Unfortunately, this approach does not allow for consideration of the effects of advances in therapeutic efficacy over the 45 years of data captured by SEER. Additionally, stage and grade classifications have subtle differences across different anatomical sites and have also evolved with temporal updates of tumor classification algorithms. Therefore, out of necessity to make consistent comparisons across all sites and over the evolution of formalized staging systems, this study is required to use very broad definitions out of necessity. However, despite these drawbacks, this project would not be possible outside of a population-level registry given the extreme rarity of ASCs. By providing a standardized and systemic characterization of the clinicoepidemiological features of ASCs against nonvariant ACs and SCCs across all major sites, this study provides the comprehensive groundwork for future comparative studies that will be required to further delineate the tumor biology of this rare and poorly understood pathology. Such research will be necessary in order to implement effective and tailored treatment interventions for patients diagnosed with these cancers.
| Supplementary Material | ▴Top |
Suppl 1. Exclusion criteria and counts of all cases, ACs, SCCs, and ASCs from SEER (1975 - 2020).
Suppl 2. Variables in analysis. Categorization reflects final variable composition.
Suppl 3. Breakdown of ASC cases in SEER (1975 - 2020), both analyzed and not analyzed.
Acknowledgments
None to declare.
Financial Disclosure
This work was supported by the National Cancer Institute (NCI) grant P30CA016056 involving the use of Roswell Park Comprehensive Cancer Center’s Pathology Network, Genomic, and Biomedical Research Informatics Shared Resources. KT was supported by US National Institutes of Health grants R37CA248018, R01CA-250412, R01CA251545, R01EB029596, as well as US Department of Defense BCRP grants W81XWH-19-1-0674 and W81XWH-19-1-0111.
Conflict of Interest
None to declare.
Informed Consent
Data release from the SEER database does not require informed patient consent.
Author Contributions
MGKB and VORS designed the study. MGKB conducted the SEER analysis. MGKB compiled the figures, and MGKB and CJR composed the tables. MGKB and VORS wrote the original draft, and all authors reviewed and edited the draft. KT supervised the project. All authors have read and agreed to the published version of the manuscript.
Data Availability
All data supporting the findings of this study are available within the article. Access to the SEER database is available via signed user agreements (https://seer.cancer.gov/data/access.html).
Abbreviations
AC: adenocarcinoma (conventional/nonvariant); ASC: adenosquamous carcinoma; CI: confidence interval; CSS: cause-specific survival; HR: hazard ratio; RS: relative survival; SD: standard deviation; SCC: squamous cell carcinoma (conventional/nonvariant); SEER: Surveillance, Epidemiology, and End Results
| References | ▴Top |
- Lokuhetty D, White VA, Watanabe R, Cree IA, World Health Organization and International Agency for Research on Cancer. Digestive system tumours. Lyon: International Agency for Research on Cancer. 2019.
- International Agency for Research on Cancer, Travis WD, Brambilla E, Burke A, Marx A, Nicholson AG. WHO classification of tumours of the lung, pleura, thymus and heart. Lyon: International Agency for Research on Cancer. 2015.
- Simone CG, Zuluaga Toro T, Chan E, Feely MM, Trevino JG, George TJ, Jr. Characteristics and outcomes of adenosquamous carcinoma of the pancreas. Gastrointest Cancer Res. 2013;6(3):75-79.
pubmed pmc - World Health Organization. International classification of diseases for oncology (ICD-O). World Health Organization, 2013
- Sapuppo E, Brunetti O, Tessitore D, Brandi G, Di Giovanni N, Fadda G, Luchini C, et al. Rare histotypes of epithelial biliary tract tumors: A literature review. Crit Rev Oncol Hematol. 2023;181:103892.
doi pubmed - Hoshimoto S, Aiura K, Shito M, Kakefuda T, Sugiura H. Adenosquamous carcinoma of the ampulla of Vater: a case report and literature review. World J Surg Oncol. 2015;13:287.
doi pubmed pmc - Maki RG. Trials and tribulations in rare cancer clinical research. J Clin Oncol. 2024;42(8):865-867.
doi pubmed pmc - Gallicchio L, Daee DL, Rotunno M, Barajas R, Fagan S, Carrick DM, Divi RL, et al. Epidemiologic research of rare cancers: trends, resources, and challenges. Cancer Epidemiol Biomarkers Prev. 2021;30(7):1305-1311.
doi pubmed pmc - Che WQ, Li YJ, Tsang CK, Wang YJ, Chen Z, Wang XY, Xu AD, et al. How to use the surveillance, epidemiology, and end results (SEER) data: research design and methodology. Mil Med Res. 2023;10(1):50.
doi pubmed pmc - Duggan MA, Anderson WF, Altekruse S, Penberthy L, Sherman ME. The surveillance, epidemiology, and end results (SEER) program and pathology: toward strengthening the critical relationship. Am J Surg Pathol. 2016;40(12):e94-e102.
doi pubmed pmc - Benesch MGK, O'Brien SBL. Epidemiology of undifferentiated carcinomas. Cancers (Basel). 2022;14(23):5819.
doi pubmed pmc - Benesch MGK, Mathieson A. Epidemiology of mucinous adenocarcinomas. Cancers (Basel). 2020;12(11):3193.
doi pubmed pmc - Benesch MGK, Mathieson A. Epidemiology of signet ring cell adenocarcinomas. Cancers (Basel). 2020;12(6):1544.
doi pubmed pmc - Benesch MGK, Nelson ED, O'Brien SBL. Malignant transformation of long-standing ileal Crohn's disease likely favors signet ring cell adenocarcinoma histology. World J Oncol. 2023;14(6):447-456.
doi pubmed pmc - Benesch MGK, Mathieson A, O'Brien SBL. Effects of tumor localization, age, and stage on the outcomes of gastric and colorectal signet ring cell adenocarcinomas. Cancers (Basel). 2023;15(3):714.
doi pubmed pmc - Benesch MGK, Nelson ED, O'Brien SBL. Location has prognostic impact on the outcome of colorectal mucinous adenocarcinomas. Cancers (Basel). 2023;16(1):147.
doi pubmed pmc - Surveillance E, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries, Nov 2021 Sub (1975-2020). National Cancer Institute, DCCPS, Surveillance Research Program, released April 2023, based on the November 2022 submission (Accessed January 2, 2024).
- Huang Z, Wang J, Zhang R, He A, Luo S, Wu R, Xiong J, et al. Pancreatic adenosquamous carcinoma: a population level analysis of epidemiological trends and prognosis. Cancer Med. 2023;12(8):9926-9936.
doi pubmed pmc - Cheng C, Luo Z, Xiong W, Shi Z, Tan H. Epidemiology and survival outcomes in adenosquamous carcinoma: a population-based study. Int J Colorectal Dis. 2022;37(7):1581-1592.
doi pubmed - Qian H, Ji X, Liu C, Dang Y, Li X, Zhang G. Clinical characteristics, prognosis, and nomogram for esophageal cancer based on adenosquamous carcinoma: a SEER database analysis. Front Oncol. 2021;11:603349.
doi pubmed pmc - Cheung R. Analysis of SEER adenosquamous carcinoma data to identify cause specific survival predictors and socioeconomic disparities. Asian Pac J Cancer Prev. 2016;17(1):347-352.
doi pubmed - Qin BD, Jiao XD, Yuan LY, Liu K, Zang YS. Adenosquamous carcinoma of the bile duct: a population-based study. Cancer Manag Res. 2018;10:439-446.
doi pubmed pmc - Iemura A, Yano H, Mizoguchi A, Kojiro M. A cholangiocellular carcinoma nude mouse strain showing histologic alteration from adenocarcinoma to squamous cell carcinoma. Cancer. 1992;70(2):415-422.
doi pubmed - Okamura K, Hayakawa H, Kuze M, Takahashi H, Kosaka A, Mizumoto R, Katsuta K. Triple carcinomas of the biliary tract associated with congenital choledochal dilatation and pancreaticobiliary maljunction. J Gastroenterol. 2000;35(6):465-471.
doi pubmed - Ochiai T, Yamamoto J, Kosuge T, Shimada K, Takayama T, Yamasaki S, Ozaki H, et al. Adenosquamous carcinoma with different morphologic and histologic components arising from the intrahepatic bile duct: report of a case. Hepatogastroenterology. 1996;43(9):663-666.
pubmed - Kardon DE, Thompson LD, Przygodzki RM, Heffess CS. Adenosquamous carcinoma of the pancreas: a clinicopathologic series of 25 cases. Mod Pathol. 2001;14(5):443-451.
doi pubmed - Li Y, Wang Z, Ajani JA, Song S. Drug resistance and cancer stem cells. Cell Commun Signal. 2021;19(1):19.
doi pubmed pmc - Arnold CR, Mangesius J, Skvortsova, II, Ganswindt U. The role of cancer stem cells in radiation resistance. Front Oncol. 2020;10:164.
doi pubmed pmc
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


