| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 13, Number 4, August 2022, pages 159-171
Epidemiology and Determinants of Survival for Primary Intestinal Non-Hodgkin Lymphoma: A Population-Based Study
Vinit Singha, Dhairya Gorb, Varsha Guptac, g, Aasems Jacobd, Doantrang Dua, Hussam Eltoukhye, Trishala Meghalf
aDepartment of Internal Medicine, Monmouth Medical Center, Long Branch, NJ 07740, USA
bDepartment of Internal Medicine, Jersey Shore University Medical Center, Neptune, NJ 07753, USA
cDepartment of Hematology-Oncology, Jersey Shore University Medical Center, Neptune, NJ 07753, USA
dDepartment of Hematology-Oncology, Leonard Lawson Cancer Center, Pikeville Medical Center, Pikeville, KY 41501, USA
eDepartment of Hematology-Oncology, Rutgers Cancer Institute of New Jersey, New Brunswick, NJ 08901, USA
fDepartment of Hematology-Oncology, Monmouth Medical Center, Long Branch, NJ 07740, USA
gCorresponding Author: Varsha Gupta, Department of Hematology-Oncology, Jersey Shore University Medical Center, Neptune, NJ 07753, USA
Manuscript submitted June 8, 2022, accepted August 13, 2022, published online August 23, 2022
Short title: Epidemiology of Primary Intestinal NHL
doi: https://doi.org/10.14740/wjon1504
| Abstract | ▴Top |
Background: Gastrointestinal tract is the most common site of extranodal non-Hodgkin lymphoma (EN-NHL). Most of the published data have been on gastric NHL with limited studies on primary intestinal non-Hodgkin lymphoma (PI-NHL) considering rare incidence. We performed epidemiological and survival analysis for PI-NHL from the Surveillance, Epidemiology, and End Results (SEER) 18 database.
Methods: A total of 9,143 PI-NHL cases of age ≥ 18 years were identified from the SEER 18 database for the period 2000 - 2015. Totally, 8,568 patients were included for survival analysis. Cause-specific survival (CSS) and overall survival (OS) analysis were done for PI-NHL and PI-diffuse large B-cell lymphoma (PI-DLBCL) using sex, age of onset, treatment, histology, stage, and year of diagnosis. Survival analysis was done by using Cox proportional hazard model and Kaplan-Meier plot with log-rank test.
Results: The percentage of PI-NHL of all the intestinal cancers and EN-NHL were 1.35%, and 10.52%, respectively. The age-adjusted incidence was 0.9145/100,000 population for the study population. PI-NHL was more common among patients aged ≥ 60 years, male and non-Hispanics Whites. Majority of patients were diagnosed at stage 1 and 2 (74%), and DLBCL (44.8%) was the most common histology. Overall median survival was 111 (95% confidence interval (CI): 105 - 117) months. In OS analysis, significant increased risk of mortality was seen with T-cell NHLs vs. DLBCL (hazard ratio (HR): 2.56), patients aged ≥ 60 vs. < 60 years (HR: 2.87), stage 4 vs. stage 1 (HR: 1.93), male vs. female (HR: 1.17), with best outcome seen in patient treated with combination of chemotherapy and surgery vs. none (HR: 0.45). Similar results were seen in CSS and for PI-DLBCL as well. Significant improvement in outcomes was observed for PI-DLBCL patients receiving chemotherapy with/without surgery.
Conclusions: Findings from our large, population-based study reveal PI-NHL is a rare type of intestinal malignancy with significant difference in survival based on histological and epidemiological characteristics.
Keywords: Primary intestinal non-Hodgkin lymphoma; Non-Hodgkin lymphoma; Intestinal cancers; SEER database and epidemiology; Lymphoma; Gastrointestinal cancer; Cancer survival
| Introduction | ▴Top |
Non-Hodgkin lymphoma (NHL), a term encompassing various neoplasms of lymphoid origin, is the most common type of blood cancer in the USA [1]. The most common site for extranodal non-Hodgkin lymphoma (EN-NHL) is the gastrointestinal tract, constituting about 30-40% of the total NHL cases. Amongst primary gastrointestinal tract lymphomas, gastric lymphoma is the most common site, followed by small intestinal lymphoma and colorectal lymphoma [2]. Among types of NHL occurring in gastrointestinal tract, B-cell lymphoma is more common than T-cell lymphomas, with most T-cell lymphoma cases occurring in the ileocolic region of the intestine.
Primary intestinal non-Hodgkin lymphoma (PI-NHL) is a rare type of malignancy comprising about 2% of all gastrointestinal malignancies, and specifically about 2% of all small intestinal and 0.2% of all large intestinal malignancies [3, 4]. In a study done by the Danish lymphoma group, it was seen that over a period of 9 years between 1983 to 1991, the annual incidence rate for PI-NHL was 0.48 per 100,000, suggesting a very low incidence rate [5]. However, there is a trend of gradual increase in the number of PI-NHL cases worldwide [2, 6]. Better diagnostic techniques, awareness about the disease, more colonoscopies and reporting have contributed to the increase in diagnoses of this disease [6, 7]. This has made it necessary to increase our focus on NHL with intestinal primary site. It should also be noted that for unknown reasons, up to 75% of primary gastrointestinal tract lymphomas in the Mediterranean region and Middle East are PI-NHL and Burkitt lymphoma (BL), which has 50-fold higher incidence in Africa predominantly presenting as obstructing lesion in the terminal ileum [8, 9].
Over the years, several studies have been conducted to understand better epidemiological and survival parameters associated with primary gastrointestinal non-Hodgkin lymphoma (PGI-NHL). However, most of these studies primarily evaluated gastric NHL as opposed to PI-NHL, due to the lesser prevalence of the latter. Further, due to an overall lower incidence of affected PI-NHL patients, most of its observational and analytical studies have a relatively smaller sample size, making it challenging to derive conclusive evidence. In addition, a lower survival rate has been observed in intestinal lymphomas compared to gastric lymphoma [10], making it crucial to analyze the factors influencing survival rates in PI-NHL independently. Here, we present the epidemiological and survival data for PI-NHL from the Surveillance, Epidemiology, and End Results (SEER) 18 databases for 2000 - 2015.
| Materials and Methods | ▴Top |
This is a registry-based retrospective study executed by analysis of data from the SEER database for the years 2000 to 2015 [11]. The population in SEER 18 represents 27.8% of the US population based on the 2010 US census. It includes cancer cases from 18 cancer registries across the USA including Alaska Natives, Greater Georgia, Connecticut, Detroit (metropolitan), Hawaii, Iowa, New Mexico, Rural Georgia, California excluding SF/SJM/LA, San Francisco-Oakland, San Jose-Monterey, Seattle, Utah, Kentucky, Los Angeles, Louisiana, New Jersey, and Atlanta (metropolitan). The SEER database is a standard for population study in the USA with a case ascertainment rate of 98%. Individual patient-level data were extracted from the SEER 18 database using SEER*Stat software (version 8.0.5; Surveillance Research Program of the National Cancer Institute).
Study population
The database was queried for all patients diagnosed with PI-NHL between 2000 to 2015 based on EN-NHL ICD-O-3 recode and primary site code for small and large intestine (primary site code C17.O - C21.8 (Supplementary Material 1, www.wjon.org)). Patients with age at diagnoses ≥ 18 were included in the study. Patients with unknown staging data, survival status, and those diagnosed after autopsy were excluded from the study (Fig. 1). Complete data collection process is described in detail here (Supplementary Materials 2, 3, www.wjon.org).
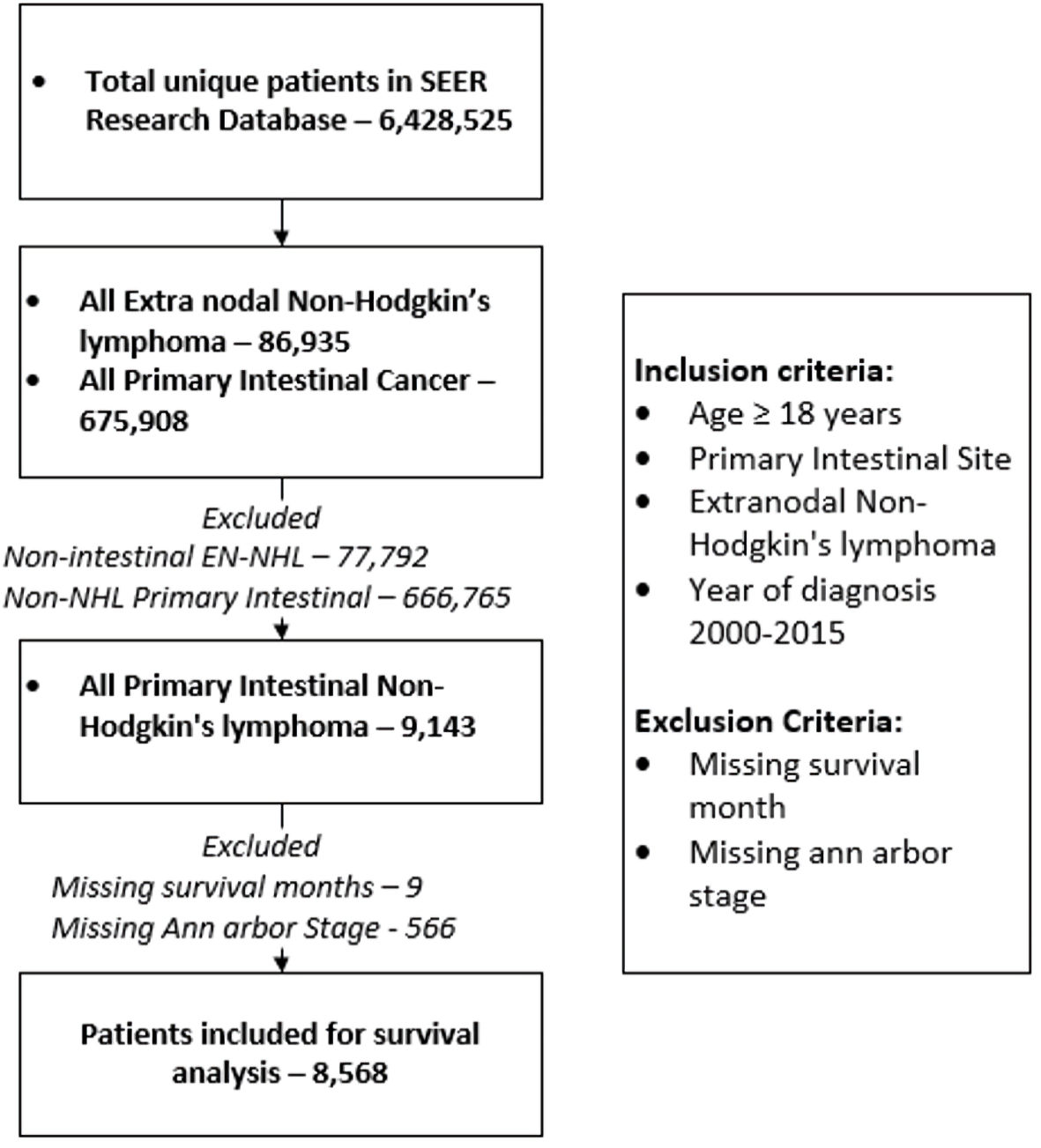 Click for large image | Figure 1. Description of patient selection and exclusion for the study. Patient listed in SEER 18 registries were included in the study. SEER: Surveillance, Epidemiology, and End Results; EN-NHL: extranodal non-Hodgkin lymphoma. |
The SEER registries collect data on patient characteristics, including age and year of diagnosis, origin, stage of diagnosis, sex, site, type of treatment, vital status, cause of death, and survival months at last follow-up or at death which were retrieved for the study. Associations between the demographic, clinical, and pathologic characteristics of patient with survival month were assessed. Age ≥ 60 is taken as a cutoff for analysis of the association between age and survival since it is considered an adverse prognostic factor as per the International Prognostic Index (IPI). pathological information included stage at diagnosis and histological type of NHL. We also included year of diagnosis as one of the determinants in the survival analysis, and it was divided into three broad periods (2000 - 2005, 2006 - 2010, and 2011 - 2015).
Statistical analysis
Descriptive statistics were used to describe patient baseline characteristics. Age-adjusted incidence rates were calculated for study duration of 2000 - 2015 for age ≥ 18 years using SEER*Stat software. Age-adjusted incidence rate ratio were calculated for sex and ethnicity (Supplementary Material 2, www.wjon.org).
Overall survival (OS) and cause-specific survival (CSS) were calculated as the time from diagnosis to death from any cause and from time of diagnosis to death from PI-NHL, respectively. Survival curves were plotted using Kaplan-Meier plots and survival analysis was performed using a log-rank test. Life tables were constructed to analyze the 1-year, 5-year and 10-year OS and CSS and were shown as percentages with a 95% confidence interval (95% CI). Patients were censored at the last follow-up in SEER, death or on December 31, 2018, whichever came first.
Hazard ratio (HR) was calculated by multivariate Cox proportional hazard model. To identify the determinant of outcome, all the covariates were analyzed in a univariate Cox proportional hazard model for OS and CSS. Those covariates which were significant for the univariate cause-specific and overall Cox proportional hazard survival model were fitted into the multivariate model to analyze the effect of each covariate independent of the others. Diffuse large B-cell lymphoma (DLBCL) was taken as reference for survival analyses considering most common type of NHL in the intestinal tract. We also calculated the survival factor for primary intestinal DLBCL considering it is the most common intestinal NHL in both small and large intestine.
All the covariates were normalized for age, sex, and staging based on significance during univariate analysis. The strength of association between each predictor and survival was expressed as a HR along with a 95% CI. All tests were two-sided, and a P value of 0.05 was considered statistically significant. All the data analyses were done in Stata/IC 16.1 and R studio with R version 4.0.1 using “survival”, “survminer” and “dplyr” packages.
Ethical approval statement
Deidentified patient data were collected from SEER database under data user agreement with NCI. Ethical review and approval were waived for this study, due to the deidentified information of the patients included in the public SEER database.
| Results | ▴Top |
A total of 675,908 patients were identified with the diagnosis of primary intestinal cancers and a total of 86,935 patients were identified to have a diagnosis EN-NHL. For the study period, 9,143 patients age ≥ 18 years were identified as PI-NHL. PI-NHL formed 1.35% (95% CI: 1.32 - 1.38) of all intestinal cancers and 10.52% (95% CI: 10.31 - 10.72) of all EN-NHL. The age-adjusted incidence of PI-NHL was 0.9145 per 100,000 individuals for age ≥ 18 for the study duration and were seen more in male and non-Hispanic White (NHW) population (Table 1). There was no significant change in the age-adjusted incidence during the study duration (Supplementary Material 4, www.wjon.org). Among the 9,143 patients with PI-NHL, 566 patients without Ann Arbor stage data and nine without survival data were excluded and finally a total of 8,568 patients were included for survival analysis (Fig. 1). We have summarized the demographic details in Table 2.
 Click to view | Table 1. Age-Adjusted Incidence for PI-NHL for Year 2000 - 2015 for Age ≥ 18 |
 Click to view | Table 2. Demography, and Clinical Characteristics of PI-NHL Patients in the Study |
Patient characteristics
PI-NHL was more common among males compared to females (60.9% vs. 39.1%). Majority (65.8%) of the PI-NHL occurred in patients aged ≥ 60 years. Majority of the patients were non-Hispanic Whites (72.83%), followed by Hispanics (11.29%) and African Americans (6.22%). More than 70% of PI-NHL were diagnosed at early stages (stages 1 and 2). The combination of surgery and chemotherapy (29.25%) was the most common approach used for the treatment. Only surgery or chemotherapy were used in 28.55% and 23.55% of patients, respectively. No intervention was done in 18.65% of the patients.
Histological classification of PINHL
The histological distribution of PI-NHL is summarized in Table 3. The majority of the PI-NHL were B-cell lymphoma, with DLBCL (44.76%) being the most common histology followed by follicular lymphoma (18.24%) and mucosa-associated lymphoid tissue lymphoma (MALToma) (13.54%). T-cell lymphoma constituted 4.58% of the total cases and 77.8% cases involved small intestine. Majority (75.8%) of follicular lymphoma had small intestine as primary site while mantle cell lymphoma (81.7%) and MALToma (61.8%) were more common in large intestine.
 Click to view | Table 3. Distribution of Histological Subtypes of Non-Hodgkin Lymphoma Across the Intestinal Tract |
Survival analysis
OS and CSS were calculated. One-year, 5-year and 10-year survival probabilities are listed in Table 4. Median survival in the overall study population was 111 months (95% CI: 105 - 117), with a nonsignificant difference with HR 1.00 (95% CI: 0.99 - 1.1, P value = 0.14) between small intestine (median survival: 117 months, 95% CI: 109 - 128 months) and large intestinal lymphomas (median survival: 103 months, 95% CI: 98 - 112 months). Survival data are summarized in Table 5. Univariate HRs for all the covariates are summarized here (Supplementary Material 5, www.wjon.org). On the multivariate analysis, compared to patients aged < 60 years, patients aged ≥ 60 years had worse cause-specific (HR: 2.13, 95 CI: 1.94 - 2.34) and overall survival (HR: 2.87, 95% CI: 2.65 - 3.10). Male sex conferred an increased risk of mortality for both CSS (HR: 1.14, 95% CI: 1.05 - 1.23) and OS (HR: 1.17, 95% CI: 1.10 - 1.24) compared to female sex. Stage 4 patients had a significantly higher risk of mortality compared to stage 1 disease (CSS HR: 2.56, 95% CI: 2.31 - 2.83; OS HR: 1.93, 95% CI: 1.79 - 2.09). Compared to DLBCL, follicular lymphoma (HR: 0.29, 95% CI: 0.26 - 0.32) and MALToma (HR: 0.37, 95% CI: 0.33 - 0.41) had better OS. T-cell lymphoma however, had the worse OS outcomes compared to DLBCL (HR: 2.56, 95% CI: 2.28 - 2.88). BL conferred poor OS compared to DLBCL with HR trending towards statistical significance (HR: 1.17, 95% CI: 1.00 - 1.38).
 Click to view | Table 4. Overall Survival and Cause-Specific Survival at 1-Year, 5-Year and 10-Year for All PI-NHL Patients |
 Click to view | Table 5. Multivariate Cox Proportional Hazard Analysis of the Survival Factors for Cause-Specific and Overall Survival for All PI-NHL Patients |
For treatment, there was no significant difference seen for OS between no treatment/observation and patients who only underwent surgery whereas CSS showed slight better outcome in patients with surgery with HR of 0.82 (95% CI: 0.73 - 0.93). Patients who underwent chemotherapy and multimodality treatment with chemotherapy and surgery had better outcome than observation or surgery alone with minimal yet significant difference between themselves for both CSS and OS as shown in Table 5. Cause-specific and overall survival of PI-NHL increased significantly over the years compared to 2000 - 2005 period. As expected, 1-year and 3-year CSS and OS probability also increased over the year (Table 6). OS and cause-specific Kaplan-Meier curves were plotted for survival factors included in Cox proportional hazard model of PI-NHL and shown in Figures 2 and 3, respectively. Survival analysis of PI-DLBCL showed similar trends as seen for broader PI-NHL and is summarized in Table 7 with OS and CSS Kaplan-Meier plots shown in Figures 4 and 5, respectively.
 Click to view | Table 6. Overall and Cause-Specific Survival Probability at 1-Year and 3-Year for Each Year of Diagnosis Group |
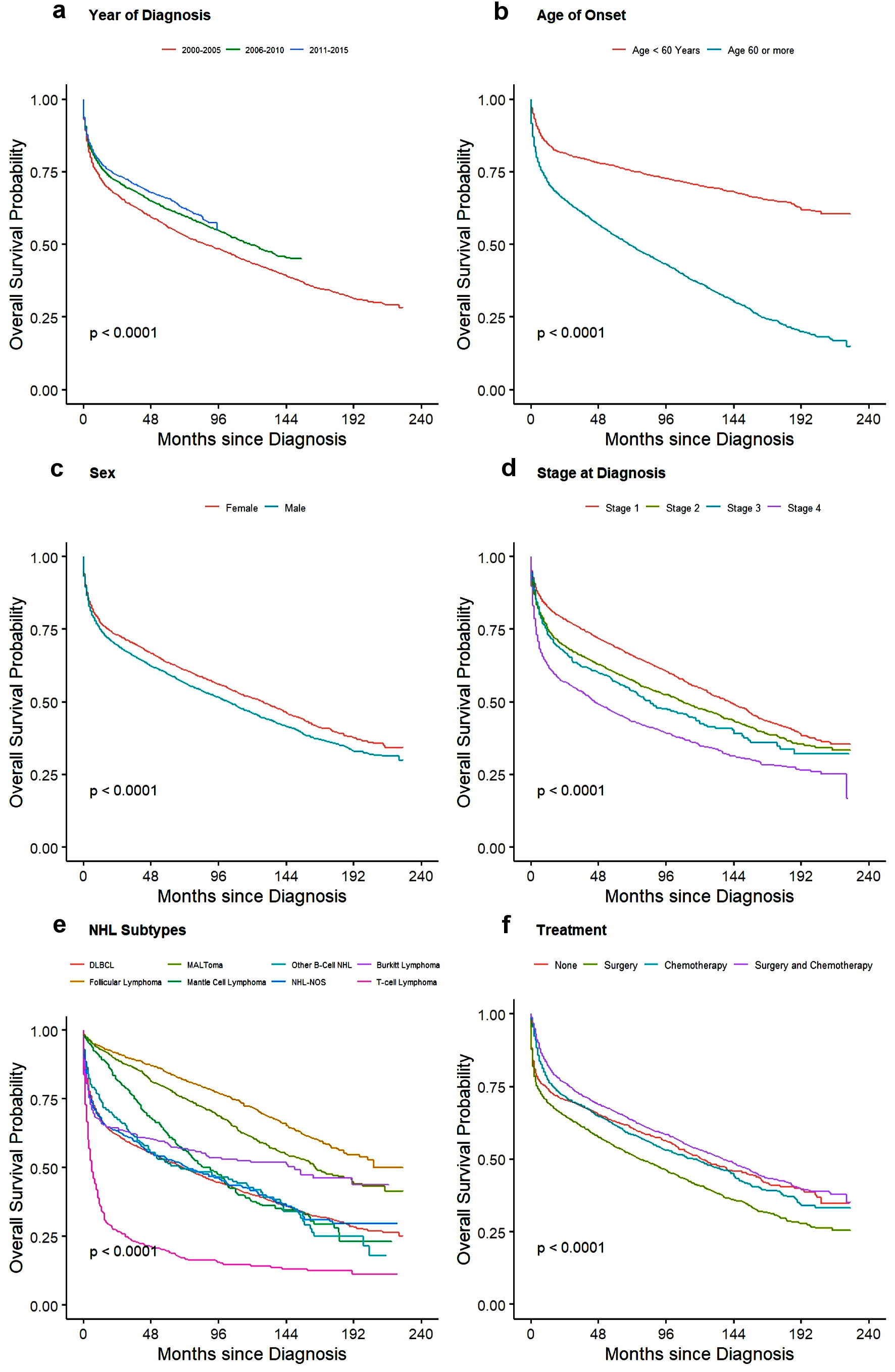 Click for large image | Figure 2. Kaplan-Meier plots for overall survival among all PI-NHL patients for effect: (a) year of diagnosis, (b) age, (c) sex, (d) stage of disease, (e) NHL subtypes, and (f) treatment. PI-NHL: primary intestinal non-Hodgkin lymphoma. |
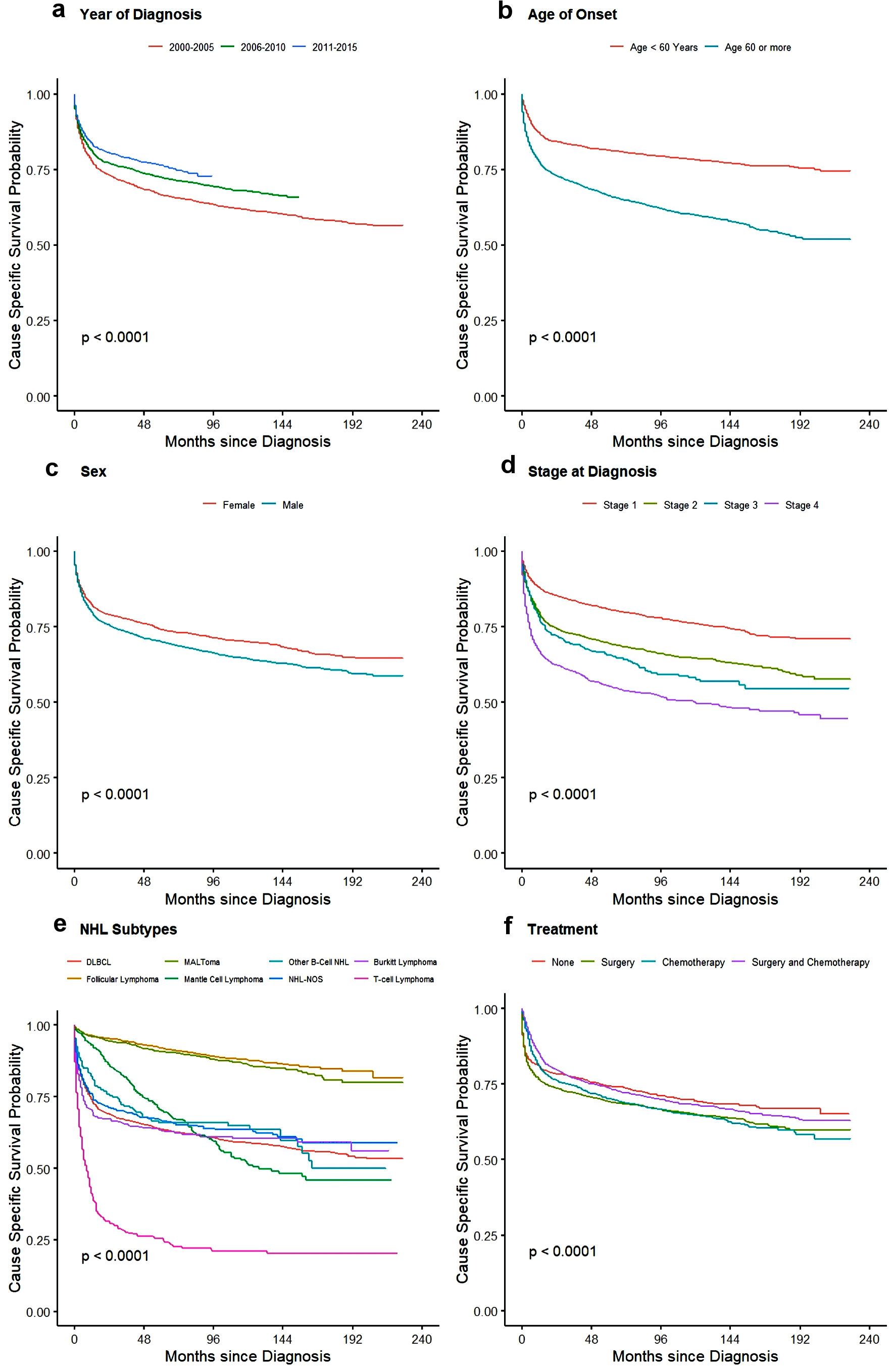 Click for large image | Figure 3. Kaplan-Meier plots for cause-specific survival among all PI-NHL patients for effect: (a) year of diagnosis, (b) age, (c) sex, (d) stage of disease, (e) NHL subtypes, and (f) treatment. PI-NHL: primary intestinal non-Hodgkin lymphoma. |
 Click to view | Table 7. Multivariate Cox Proportional Hazard Analysis of the Survival Factors for Cause-Specific and Overall Survival for PI-DLBCL Patients |
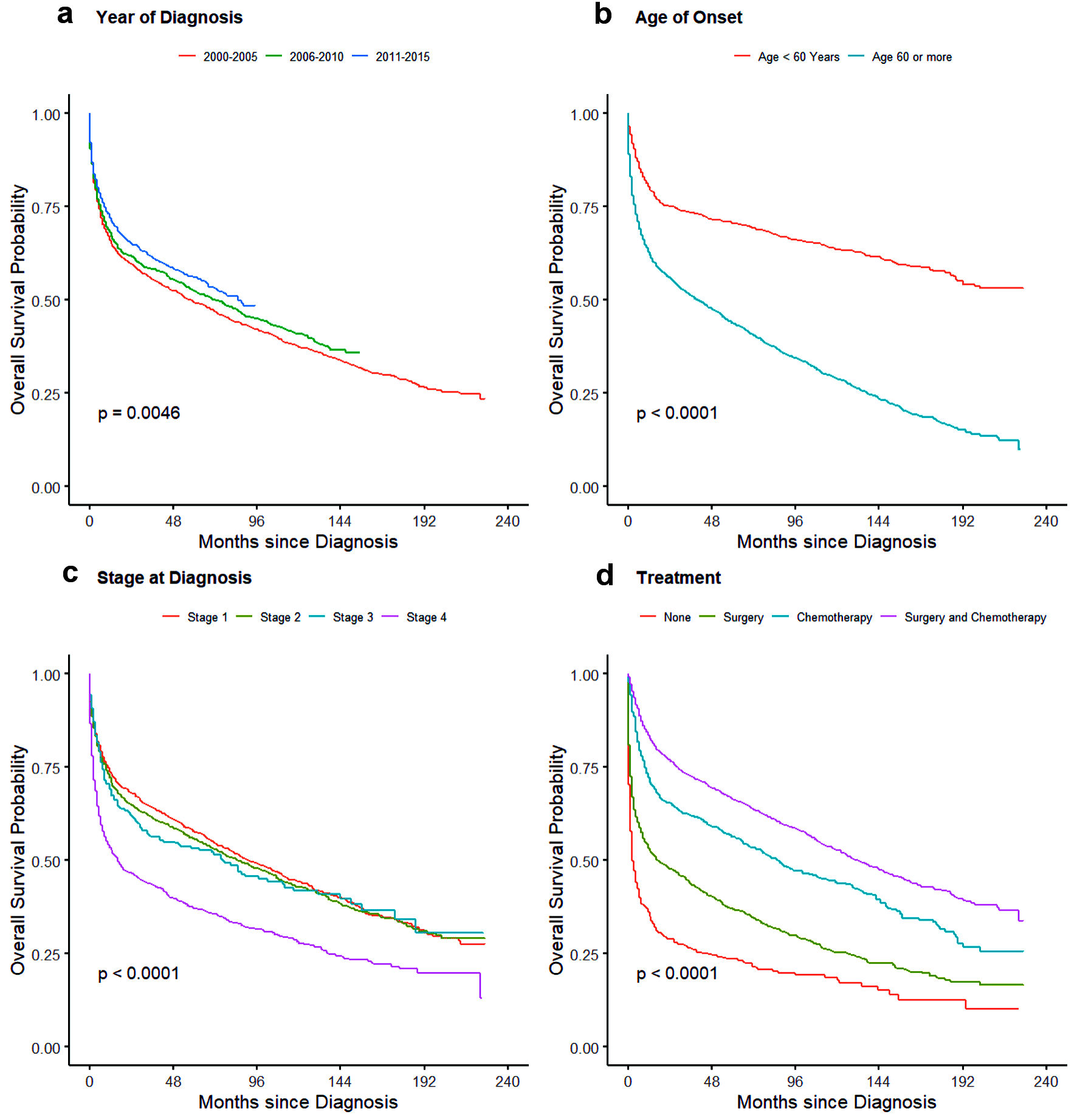 Click for large image | Figure 4. Kaplan-Meier plots for overall survival probability for primary intestinal DLBCL for effects: (a) year of diagnosis, (b) age, (d) stage of disease, and (d) treatment. DLBCL: diffuse large B-cell lymphoma. |
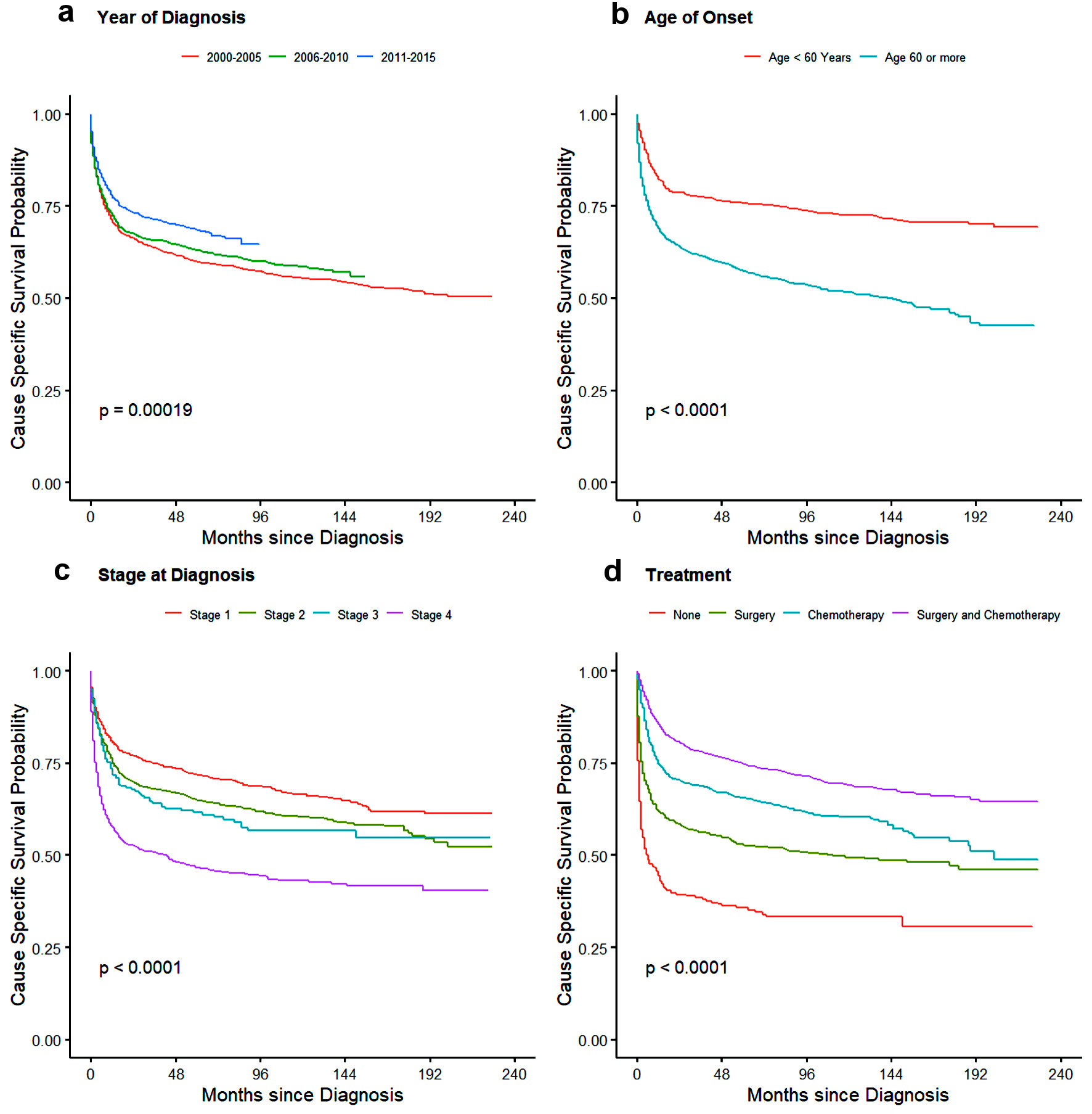 Click for large image | Figure 5. Kaplan-Meier plot showing cause-specific survival probability for PI-DLBCL for effects: (a) year of diagnosis, (b) age, (d) stage of disease, and (d) treatment. PI-DLBCL: primary intestinal diffuse large B-cell lymphoma |
| Discussion | ▴Top |
Although PI-NHL is a rare cancer, it accounts for a considerable portion of EN-NHL. The SEER database of the National Cancer Institute which enlists data for cancer patients representing 27.8% of the US population is an excellent resource for doing a large population-based study for this rare entity. To the best of our knowledge, our study with more than 9,000 PI-NHL patients is the largest analysis of clinical characteristics and outcomes among patients with PI-NHL.
Although the incidence of PI-NHL has remained relatively same over the years of analysis between 2000 to 2015, CSS and OS have improved significantly. This may be attributed to the improvement in diagnosis and treatment of lymphoma in general. In our study, 65.77% of patients were aged ≥ 60 years with the median age of onset being 66.5 years (95% CI: 55 - 77). The higher prevalence of the condition in patients > 60 years with PI-NHL was more common among males in congruence with previous data. This is the only study which has reported population-based age-adjusted incidence rate with incidence rate ratio close to twice for male compared to female, 0.62 times for non-Hispanic Black and 0.92 times for Hispanic patients compared to non-Hispanic White population. In the previous studies, the number of reported cases were almost twice in the males for the eastern Asian population to 1.5 times in German and North American populations, which were seen in our patient group as well [10, 12, 13].
On multivariate analysis, age, sex, stage of disease, histology, and type of treatment were found to be the factors affecting OS. Age of onset and stage are currently parts of the IPI scoring for NHL. In our study, patients older than 60 years were at twice the risk of cause-specific mortality, whereas OS was even more strongly dependent on age with 2.68 times worse outcome in this group. In a study done on the Medicare beneficiaries with newly diagnosed NHLs [14], it was seen that NHL-specific mortality increases over the age for all types of NHLs and is more influenced by the comorbidities for younger individuals than that of older patients. The presence of comorbidities influences mortality from other conditions. In terms of sex, male patients have higher risk of both cause-specific and overall mortality for unknown reasons.
We did not observe any significant difference in outcome between the small and large intestine primary sites. DLBCL is the most common subtype of PI-NHL, followed by follicular lymphoma and MALToma. DLBCL and BL had similar incidence in small and large intestine, whereas MALToma and Mantle cell lymphoma were more common in the large intestine (Table 2). Similar findings were seen in previous studies comprising data from multiple centers [7, 12 13]. The median OS of primary intestinal T-cell lymphoma, PI-DLBCL and MALToma was 6 months, 71 months, and 165 months, respectively. Median survival was not reached in follicular lymphoma as more than 50% patients (in our study, 71.84% of total follicular lymphoma) survived beyond the study cut-off date. This finding is consistent with the recent study, where median survival was not reached in the case of follicular lymphoma even after following up of 20 years [15]. CSS was 8 months in T-cell lymphoma indicating the disease as cause of death in majority of patients, while the median CSS was not reached in other common histology.
Treatment of PI-NHL varies based on histologic subtypes, clinical presentation, stage, and site. In our study, we found that 19% (n = 1,598) of patients did not receive any treatment and out of those 54.51% (871/1,598) of the patients had indolent NHL (Supplemental Material 6, www.wjon.org). Indolent NHL like follicular lymphoma and MALToma can be managed by observation alone in many cases, especially early stages [16]. It is known that for indolent lymphomas systemic therapy is indicated with progression of disease or worsening of symptoms, mostly at later stages. In those cases, OS is superior for patients who received treatment when compared to those who were unable to receive treatment because of various reasons. Patients with aggressive lymphoma usually present with complicated clinical presentation or poor performance status which might make them non-amenable to get further intervention. There is a possibility that those patients had advanced disease so they decided to pursue comfort care and might explain worse outcome in the “no” treatment group. Chemotherapy is the primary modality of treatment for all types of NHL and holds true for intestinal NHL. In our patient population, more than half of all patients and more than half of patients who underwent surgery received chemotherapy, as seen in a recent study published on small intestinal lymphoma utilizing the National Cancer Database [17]. This could indicate that many PI-NHL presents with intestinal mass and symptoms of obstruction, which necessitates the use of surgical intervention for immediate management. In a report published from China, around 48% of patients with PI-NHL required surgical intervention [13]. In a recent report published from the SEER database on duodenal NHL, there has been a decline in the proportion of patients requiring surgery for duodenal NHL. In our study, which incorporated small and large intestine NHL, with around 60% of patients required surgical intervention.
Patients who underwent multimodality treatment with surgery and chemotherapy had better OS in univariate and multivariate analysis. Whereas, on univariate survival analysis (Figs. 2f, 3f), patients with “no” treatment were observed to have better survival compared to patients undergoing surgery, and no statistical difference for patients undergoing chemotherapy alone. These findings of better survival in “no” treatment group might have been because of extended long-term survival in “no” treatment group in patients with indolent lymphomas requiring very late intervention with progression of disease and better survival in elderly managed with observation alone [16, 18]. On multivariate analysis, CSS and OS benefit of treatment strategies were very evident with better survival in all treatment groups except OS for patients undergoing surgery alone (Table 5). In a retrospective study which showed similar outcomes, it was observed that patients receiving surgery and chemotherapy were younger, male, and patients with fewer comorbidities [19-21]. Another important reason that was highlighted for better survival in this group compared to those receiving chemotherapy alone, is the low local relapse rate in intestinal B-cell lymphoma [12].
Similar findings in survival analysis were also noted in our separate analysis of PI-DLBCL. Effects of treatment was more evident here with patients receiving chemotherapy with or without surgery having better CSS and OS compared to those who received surgery alone. Patient older than 60 years had worse outcomes compared to younger patients. Since the benefit of chemotherapy is evident in these patients, comorbidities, age, and surgical complications could have precluded them from receiving systemic chemotherapy contributing to the inferior survival. The overall outcomes have improved in recent years (2011 - 2015) and could be from introduction of rituximab, use of tolerable chemotherapy regimens, better management of chemotherapy-related complications and also because of better understanding of disease biology over the years [22]. Significant improvements in diagnosis, staging, and response assessment using PET scan, as per Lugano classification, might have also contributed to this improvement [23].
Considering the rare occurrence of this subtype of intestinal cancer, large population-based database like SEER database has been utilized to provide conclusive epidemiological data. However, our study has certain limitations. This is a retrospective study with registry data lacking detailed analysis of clinical characteristics, biomarkers including cytogenetics data, and details of chemotherapy used, whether it was a combination of chemotherapy and immunotherapy or only chemotherapy. There are various chemotherapy regimens approved for NHL and SEER database does not provide the exact regimen used or if patients got any maintenance therapy. There is also a lack of details on the type of surgery done and clinical presentation leading to choosing surgery as an option over chemoimmunotherapy. Similarly cause of death data which is collected from death certificate in ordered to identify single, disease-specific cause of death has some limitations with risk of misattributions [24]. It has been also seen that SEER database has limited utility with low sensitivity in identifying cancer treatment which varied by cancer site, stage, and patient characteristics [25]. Although positive predictive value was > 85% for majority of treatment and cancer type which has improved over the time in recent studies [26]. In our study, we did a very broad analysis covering all the subtypes of NHL together and over 15 years. At the same time, as DLBCL was the most common subtype of NHL diagnosed within this population and because of its unique behavior, we did sub-analysis of DLBCL separately, looking into its specific behavior and survival. There are further studies needed considering each histology of NHL separately, as NHL is a very broad disease entity and behavior of indolent lymphomas differ remarkably from aggressive B cell lymphomas.
Conclusions
This is the largest study reported on PI-NHL and summarizes the epidemiology of the PI-NHL and factors that influence survival. Our data suggest that the evaluation of PI-NHL based on epidemiology, site, and histological subtype remains very informative and very much needed. In our study we found that PI-NHL is mainly a disease of old age with higher incidence in non-Hispanic White, and male patient. Male sex, late-stage tumors, and certain histologic types including T-cell lymphoma, BL, and DLBCL are associated with poor outcomes. We also concluded that the outcome of combination of surgery and chemotherapy is not significantly different from chemotherapy alone but has better outcome than surgery or observation/no treatment. Further studies conducted by multicenter collaboration encompassing important details along with biomarkers will add lots of value to this subtype on NHL and rare type of intestinal cancer. A similar study with detailed data collection from SEER-Medicare database would also help in bridging some of the gap as well.
| Supplementary Material | ▴Top |
Suppl 1. WHO-ICD-O primary site codes and distribution of PINHL across the intestinal tract.
Suppl 2. Selection algorithm used for calculating age-adjusted incidence of primary intestinal extranodal non-Hodgkin lymphoma in Rate session of SEER Stat.
Suppl 3. Selection algorithm used for identifying primary intestinal extranodal non-Hodgkin lymphoma in Case Listing session of SEER Stat.
Suppl 4. Annual age-adjusted incidence rate from year 2000 - 2015; adjusted for 2000 US standard population (standard population for study population: 203,852,188).
Suppl 5. Univariate analysis of the survival factors for cause-specific and overall survival for all PINHL patients.
Suppl 6. Stage and treatment distribution of each histological subtype of PINHL.
Acknowledgments
None to declare.
Financial Disclosure
There was no specific funding source to be mentioned.
Conflict of Interest
The authors declare that they have no competing interest.
Informed Consent
Not applicable.
Author Contributions
Conceptualization: VS, VG, AJ, and TM. Methodology: VS, DG, VG, AJ, DD, HE and TM. Data curation: VS, VG, and AJ. Formal analysis: VS, VG, AJ, and DG. Original draft preparation: VS, DG, VG, AJ, DD, HE, and TM. Writing-review and editing: VS, DG, VG, AJ, DD, HE, and TM. All authors have contributed equally, read and agreed to the published version of the manuscript.
Data Availability
All data were extracted from and available on the Surveillance, Epidemiology, and End Results Program (https://seer.cancer.gov/), accessed on 10 September 2021.
Abbreviations
BL: Burkitt lymphoma; CSS: cause-specific survival; DLBCL: diffuse large B-cell lymphoma; EN-NHL: extranodal non-Hodgkin lymphoma; HR: hazard ratio; IPI: International Prognostic Index; MALToma: mucosa-associated lymphoid tissue lymphoma; NHL: non-Hodgkin lymphoma; NHW: non-Hispanic White; OS: overall survival; PGI-NHL: primary gastrointestinal non-Hodgkin lymphoma; PI-DLBCL: primary intestinal diffuse large B-cell lymphoma; SEER: Surveillance, Epidemiology, and End Results
| References | ▴Top |
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
doi pubmed - Groves FD, Linet MS, Travis LB, Devesa SS. Cancer surveillance series: non-Hodgkin's lymphoma incidence by histologic subtype in the United States from 1978 through 1995. J Natl Cancer Inst. 2000;92(15):1240-1251.
doi pubmed - Ghimire P, Wu GY, Zhu L. Primary gastrointestinal lymphoma. World J Gastroenterol. 2011;17(6):697-707.
doi pubmed - Lightner AL, Shannon E, Gibbons MM, Russell MM. Primary gastrointestinal non-Hodgkin's lymphoma of the small and large intestines: a systematic review. J Gastrointest Surg. 2016;20(4):827-839.
doi pubmed - d'Amore F, Brincker H, Gronbaek K, Thorling K, Pedersen M, Jensen MK, Andersen E, et al. Non-Hodgkin's lymphoma of the gastrointestinal tract: a population-based analysis of incidence, geographic distribution, clinicopathologic presentation features, and prognosis. Danish Lymphoma Study Group. J Clin Oncol. 1994;12(8):1673-1684.
doi pubmed - Wang GB, Xu GL, Luo GY, Shan HB, Li Y, Gao XY, Li JJ, et al. Primary intestinal non-Hodgkin's lymphoma: a clinicopathologic analysis of 81 patients. World J Gastroenterol. 2011;17(41):4625-4631.
doi pubmed - Howell JM, Auer-Grzesiak I, Zhang J, Andrews CN, Stewart D, Urbanski SJ. Increasing incidence rates, distribution and histological characteristics of primary gastrointestinal non-Hodgkin lymphoma in a North American population. Can J Gastroenterol. 2012;26(7):452-456.
doi pubmed - Al-Saleem T, Al-Mondhiry H. Immunoproliferative small intestinal disease (IPSID): a model for mature B-cell neoplasms. Blood. 2005;105(6):2274-2280.
doi pubmed - Ogwang MD, Bhatia K, Biggar RJ, Mbulaiteye SM. Incidence and geographic distribution of endemic Burkitt lymphoma in northern Uganda revisited. Int J Cancer. 2008;123(11):2658-2663.
doi pubmed - Daum S, Ullrich R, Heise W, Dederke B, Foss HD, Stein H, Thiel E, et al. Intestinal non-Hodgkin's lymphoma: a multicenter prospective clinical study from the German Study Group on Intestinal non-Hodgkin's Lymphoma. J Clin Oncol. 2003;21(14):2740-2746.
doi pubmed - Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Incidence - SEER Research Data, 18 Registries, Nov 2020 Sub (2000-2018) - linked to county attributes - time dependent (1990-2018) income/rurality, 1969-2.
- Kim SJ, Kang HJ, Kim JS, Oh SY, Choi CW, Lee SI, Won JH, et al. Comparison of treatment strategies for patients with intestinal diffuse large B-cell lymphoma: surgical resection followed by chemotherapy versus chemotherapy alone. Blood. 2011;117(6):1958-1965.
doi pubmed - Zheng G, Wang Y, Zhao Y, Zheng Z. Clinicopathological features, treatment strategy, and prognosis of primary non-Hodgkin's lymphoma of the duodenum: a SEER database analysis. Can J Gastroenterol Hepatol. 2020;2020:9327868.
doi pubmed - Hester LL, Park SI, Wood WA, Sturmer T, Brookhart MA, Lund JL. Cause-specific mortality among Medicare beneficiaries with newly diagnosed non-Hodgkin lymphoma subtypes. Cancer. 2019;125(7):1101-1112.
doi pubmed - Batlevi CL, Sha F, Alperovich A, Ni A, Smith K, Ying Z, Soumerai JD, et al. Follicular lymphoma in the modern era: survival, treatment outcomes, and identification of high-risk subgroups. Blood Cancer J. 2020;10(7):74.
doi pubmed - Schmatz AI, Streubel B, Kretschmer-Chott E, Puspok A, Jager U, Mannhalter C, Tiemann M, et al. Primary follicular lymphoma of the duodenum is a distinct mucosal/submucosal variant of follicular lymphoma: a retrospective study of 63 cases. J Clin Oncol. 2011;29(11):1445-1451.
doi pubmed - Lu PW, Fields AC, Yoo J, Irani J, Goldberg JE, Bleday R, Melnitchouk N. Surgical management of small bowel lymphoma. J Gastrointest Surg. 2021;25(3):757-765.
doi pubmed - Lumish M, Falchi L, Imber BS, Scordo M, von Keudell G, Joffe E. How we treat mature B-cell neoplasms (indolent B-cell lymphomas). J Hematol Oncol. 2021;14(1):5.
doi pubmed - Hong YW, Kuo IM, Liu YY, Yeh TS. The role of surgical management in primary small bowel lymphoma: A single-center experience. Eur J Surg Oncol. 2017;43(10):1886-1893.
doi pubmed - Lee J, Kim WS, Kim K, Ahn JS, Jung CW, Lim HY, Kang WK, et al. Prospective clinical study of surgical resection followed by CHOP in localized intestinal diffuse large B cell lymphoma. Leuk Res. 2007;31(3):359-364.
doi pubmed - Lewin KJ, Ranchod M, Dorfman RF. Lymphomas of the gastrointestinal tract: a study of 117 cases presenting with gastrointestinal disease. Cancer. 1978;42(2):693-707.
doi - Coiffier B, Thieblemont C, Van Den Neste E, Lepeu G, Plantier I, Castaigne S, Lefort S, et al. Long-term outcome of patients in the LNH-98.5 trial, the first randomized study comparing rituximab-CHOP to standard CHOP chemotherapy in DLBCL patients: a study by the Groupe d'Etudes des Lymphomes de l'Adulte. Blood. 2010;116(12):2040-2045.
doi pubmed - Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, Lister TA, et al. Recommendations for initial evaluation, staging, and response assessment of Hodgkin and non-Hodgkin lymphoma: the Lugano classification. J Clin Oncol. 2014;32(27):3059-3068.
doi pubmed - Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J Natl Cancer Inst. 2010;102(20):1584-1598.
doi pubmed - Noone AM, Lund JL, Mariotto A, Cronin K, McNeel T, Deapen D, Warren JL. Comparison of SEER treatment data with medicare claims. Med Care. 2016;54(9):e55-64.
doi pubmed - Kraus RD, Hamilton AS, Carlos M, Ballas LK. Using hospital medical record data to assess the accuracy of the SEER Los Angeles Cancer Surveillance Program for initial treatment of prostate cancer: a small pilot study. Cancer Causes Control. 2018;29(9):815-821.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


