| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Original Article
Volume 14, Number 2, April 2023, pages 125-134
Overall Survival Rates Become Similar Between Percutaneous Ablation and Hepatic Resection With Increasing Age Among Elderly Patients With Early Hepatocellular Carcinoma
Hong Liang Zoua, e, Hui Tangb, e, Chao Ana, e, Lu Jun Shena, e, Ji Bin Lic, Wan Yee Laud, Yi Quan Jianga, b, f, Jin Hua Huanga, f
aDepartment of Minimally Invasive Interventional Therapy, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China
bDepartment of Hepatic Surgery and Liver Transplantation Center of the Third Affiliated Hospital, Organ Transplantation Institute, Sun Yat-sen University, Guangzhou 510060, China
cDepartment of Clinical Research, Sun Yat-sen University Cancer Center; State Key Laboratory of Oncology in South China; Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, China
dFaculty of Medicine, The Chinese University of Hong Kong, Shatin, N.T., Hong Kong SAR, China
eThese authors contributed equally to this article.
fCorresponding Author: Jin Hua Huang and Yi Quan Jiang, Collaborative Innovation Center for Cancer Medicine, Guangzhou 510060, Chinaand
Manuscript submitted January 16, 2023, accepted March 2, 2023, published online March 24, 2023
Short title: Treatment of HCC in Elderly Patients
doi: https://doi.org/10.14740/wjon1479
| Abstract | ▴Top |
Background: This study aimed to investigate the efficacy and safety of percutaneous ablation versus hepatectomy in an elderly population with hepatocellular carcinoma (HCC).
Methods: Retrospective data on patients aged ≥ 65 years with very-early/early stages of HCC (≤ 50 mm) were obtained from three centers in China. Inverse probability of treatment weighting analysis was performed after stratifying the patients by age (65 - 69, 70 - 74 and ≥ 75 years).
Results: Of the 1,145 patients, 561 and 584 underwent resection and ablation, respectively. For patients aged 65 - 69 and 70 - 74 years, resection resulted in significantly better overall survival (OS) than ablation (age 65 - 69, P < 0.001, hazard ratio (HR) = 0.27; age 70 - 74, P = 0.012, HR = 0.64). However, in patients aged ≥ 75 years, resection and ablation resulted in a similar OS (P = 0.44, HR = 0.84). An interactive effect existed between treatment and age (effect of treatment on OS, age 65 - 69 as the reference, for age 70 - 74, P = 0.039; for age ≥ 75, P = 0.002). The HCC-related death rate was higher in patients aged 65 - 69, and the liver/other cause-related death rate was higher in patients aged > 69. Multivariate analyses showed that the type of treatment, number of tumors, α-fetoprotein level, serum albumin level and associated diabetes mellitus were independent factors associated with OS, but not hypertension or heart diseases.
Conclusion: With increasing patient age, the treatment outcomes of ablation become similar to those of resection. A higher liver/other cause-related death rate in very elderly patients may shorten the life expectancy, which may lead to the same OS regardless of whether resection or ablation is chosen.
Keywords: Hepatocellular carcinoma; Hepatectomy; Ablation; Elderly; Treatment decision-making
| Introduction | ▴Top |
Liver cancer is the sixth most common malignancy in the world and the fourth leading cause of death from malignant tumors [1]. An epidemiological report published in 2018 showed that approximately 841,000 patients were diagnosed with liver cancer and approximately 782,000 died each year [2]. The World Health Organization estimates that more than 1 million patients will die of liver cancer annually by 2030. Hepatocellular carcinoma (HCC) is the most common type of liver cancer, accounting for approximately 75% to 85% of all cases [3].
With advances in medicine and population aging in most developed countries, the number of elderly patients is rapidly increasing [4]. The characteristics of cancer patients are notably different between elderly and young populations. While elderly cancer patients usually present with more comorbid diseases [5], their immune system is not as strong as that of young cancer patients, leading to significant differences in antitumor immunity and tumor microenvironments [6]. These characteristics suggest that cancer treatment strategies may differ between elderly and young populations.
At present, treatment recommendations for elderly patients with very-early/early stages of HCC are still controversial [7, 8]. In general, patients with very-early/early stages of HCC are usually offered curative-intention treatments, which include partial hepatectomy, ablation, or liver transplantation. As liver transplantation is less commonly offered to elderly patients in many centers, hepatic resection and ablation are the two most common primary treatments with curative intent offered to elderly patients with HCC. Ablation is less invasive than hepatic resection [9-11], leading to increasing numbers of elderly patients undergoing ablation [12]. However, the long-term overall survival (OS) outcomes between ablation and partial hepatectomy are controversial. On the one hand, elderly patients with HCC, especially those with a tumor size less than 3 cm, have been reported to have better OS with hepatectomy than with ablation [7]. On the other hand, ablation has also been reported to result in similar OS as hepatectomy in elderly patients with a tumor size of less than 3 cm [8, 13]. Thus, more studies are urgently needed to compare the long-term OS outcomes between resection and ablation in the elderly population. The aim of this study was to determine the optimal treatment strategy for elderly patients with HCC who are candidates for curative treatment using ablation or partial hepatectomy.
| Materials and Methods | ▴Top |
Study design
Clinical data on patients who were treated between 2007 and 2017 at Sun Yat-sen University Cancer Center, Chinese PLA General Hospital and the Third Affiliated Hospital of Sun Yat-sen University were collected and analyzed. The inclusion criteria were patients with the following: 1) a histological diagnosis of HCC; 2) hepatic resection or ablation as the primary curative therapy; 3) age ≥ 65 years; and 4) a single tumor ≤ 5 cm or no more than three tumors ≤ 3 cm each. The exclusion criteria were patients with the following: 1) major vascular invasion, lymph node metastasis or distant metastasis; 2) coexisting severe organ dysfunction with a short life expectancy; 3) severe infection or sepsis; and 4) other malignant diseases. This study was approved by the relevant institutional review boards (IRBs). Patient consent for inclusion was waived by the IRBs because of the retrospective nature of the study. The study was conducted in compliance with the ethical standards of the responsible institution on human subjects as well as with the Helsinki Declaration.
Diagnosis and selection of treatment
Patients in the resection group were diagnosed with HCC based on a histological study of the resected surgical specimens. Among 584 patients who underwent ablation, 437 (74.8%) patients were diagnosed with HCC based on histological specimens obtained from biopsy, and 147 (25.2%) patients were diagnosed with HCC based on typical features of HCC on contrast-enhanced images and/or an α-fetoprotein (AFP) level higher than 400 ng/mL. The treatment strategies were obtained after discussion with the patients by a multidisciplinary treatment team composed of surgeons and minimally invasive treatment radiologists. After providing patients with information, the final treatment decision was made together by the patients and the clinicians.
Data collection
The following data were collected. 1) Baseline data included age, sex, underlying liver diseases, diabetes, hypertension, heart diseases, and type of treatment. A coexisting heart disease was defined as a history of coronary or valvular heart diseases. 2) Tumor status included tumor size, tumor number and α-fetoprotein (AFP) level. 3) Liver/kidney functional status included aspartate aminotransferase (AST) level, alanine aminotransferase (ALT) level, prothrombin time (PT), international normalized ratio (INR), platelet (PLT) count, total bilirubin (TBIL) level, serum creatinine (SCr) level and Child-Pugh grading. 4) Endpoint variables included total cost for HCC treatment, length of in-hospital stay, rate of intensive care unit admission, number of hospitalizations, 30-day mortality, 90-day mortality, recurrence status and survival status. Recurrence-free survival (RFS) was defined as the time from surgery to local, regional, or distant cancer relapse. OS was defined as the time from surgery to any cause of death. HCC-associated death was defined as death attributed to HCC. Liver-associated death was defined as death attributed to comorbidities related to hepatic failure, such as hepatic encephalopathy and esophageal and gastric variceal bleeding. Death attributed to other causes included cardiovascular and cerebrovascular diseases, Alzheimer’s disease and infection.
Hepatic resection
Hepatic resection was performed by an open approach under general anesthesia. Surgeons from the two centers with 19 to 27 years of experience in hepatectomy performed the surgeries. Intraoperative ultrasonography was routinely used. Anatomical resections in the form of segmentectomy and/or subsegmentectomy as described by Makuuchi et al were the preferred procedures [14]. Whole liver segment(s)/subsegment(s) that contained the tumor were resected. For nonanatomical resection, at least a 1 cm tumor-free margin was aimed. Pringle’s maneuver with cycles of clamping and unclamping of 10 and 5 min was routinely used.
Computed tomography (CT)-guided tumor ablation
In the current study, CT-guided percutaneous thermal ablation included radiofrequency ablation (RFA) and microwave ablation (MWA). All ablative procedures were performed percutaneously under CT guidance (Siemens, Munich, Germany) carried out by one of the senior interventional radiologists (all interventional radiologists had at least 10 years of experience with ablation). Patients were administered moderate amounts of intravenous sedation and local anesthesia. General anesthesia was administered when the patient requested it. The puncture site was anesthetized with 2% lidocaine, and a single/clustered needle electrode(s) with a 2, 3 or 4 cm exposed tip was inserted into the target tumor via the determined puncture path. A routine CT scan was performed to ensure the correct location of the guided needle. After confirmation of the location of the electrode probe, MWA or RFA was performed. The energy deposition algorithm was applied based on the manufacturers’ recommended protocols. One to three ablation sites per lesion were used to ensure complete destruction of the entire targeted tumor with a safety margin of surrounding tissues. After the ablative procedure, the electrode probe was removed, and a final CT scan was performed to reexamine the ablation zone. The ablation procedure was considered complete when the ablation zone was large enough to cover the tumor with an ablative margin of at least 1 cm.
Follow-up
Patients were followed up at 1 month after hepatectomy or ablation, once every 3 months for the first 2 years, and then once every 4 to 6 months thereafter. At each follow-up visit, in addition to history taking and physical examination, AFP, AST, ALT, PT, INR, PLT, TBIL, and SCr were analyzed, and abdominal ultrasonography, and contrast-enhanced CT or MRI were carried out.
Subgroup analysis
Patients were stratified by age into the following subgroups: 65 - 69, 70 - 74, and ≥ 75. In each age subgroup, patients with solitary tumors were further stratified by tumor size into 0 - 20 mm, 21 - 30 mm and 31 - 50 mm. A cutoff value of 30 mm was chosen in the current study based on the discrepancies regarding the choice between resection and ablation in the elderly population, as reported in two studies [7, 8]. Additionally, a cutoff value of 20 mm was chosen based on the controversy of whether RFA [15, 16] or liver resection was a more appropriate treatment for patients with a single tumor < 20 mm in size [17].
Comparison of other related factors
The total cost for HCC treatment, total in-hospital stay for HCC treatment (calculated by adding all in-hospital days of all hospitalizations), total number of hospitalizations for HCC treatment, duration of the treatment procedure in minutes and rate of intensive care unit admission after treatment were compared between the groups.
Statistical analysis
Inverse probability of treatment weighting (IPTW) was used in the comparison analysis, including subgroup analyses, between the two treatment groups. The propensity score was calculated using logistic regression on variables that were potentially associated with prognosis or that were unbalanced between the two groups in terms of age, sex, underlying liver diseases, liver cirrhosis, diabetes, hypertension, heart diseases, tumor size, tumor number, AFP level, AST level, ALT level, PT, INR, PLT count, TBIL level, serum creatinine level and Child-Pugh grading. Comparison analyses in the current study were performed after weighting the individual contributions by the inverse of the probability (calculated by the logistic regression model as mentioned above) in the groups.
The characteristics of patients who underwent ablation and resection were compared using the Chi-squared test (Fisher’s exact test for small sample) for categorical variables and Student’s t-test for continuous variables. Heterogeneity of treatment effects was assessed with tests of interaction between treatments (ablation vs. resection) and age groups (65 - 69, 70 - 74 and ≥ 75). Hazard ratios (HRs) and 95% confidence intervals (CIs) of survival in response to treatment across the different age groups were estimated. Multivariate analyses were performed using Cox proportional hazards regression analysis with a stepwise backward process. The Kaplan-Meier method with a log-rank test was applied to compare the survival outcomes of patients. An estimation of the median follow-up time was proposed with the reverse Kaplan-Meier method. All statistical tests were two-sided, and P < 0.05 was considered statistically significant. For all analyses, HRs with 95% CIs were calculated. All statistical analyses were performed using SPSS 23.0 for Windows (SPSS, Chicago, IL, USA) and R software (version 3.6.2) with the rms, survival, ggplot2, survminer, IPWsurvival and MatchIt packages.
| Results | ▴Top |
In total, 18,233 patients with HCC were managed at the study centers during the study period. Of these patients, 2,831 (15.5%) were ≥ 65 years of age, and 1,205 (42.5%) of the elderly patients underwent curative treatment in the form of resection or ablation. There were 1,145 patients (95.0%) who met the inclusion criteria; 561 underwent hepatic resection, while 584 patients underwent ablation as the primary curative therapy. In total, 769 of the patients were from Sun Yat-sen University Cancer Center, 303 patients were from Chinese PLA General Hospital, and 73 patients were from the Third Affiliated Hospital of Sun Yat-sen University. The majority of these patients were in Child-Pugh class A (95.5%), while the remaining patients were in Child-Pugh class B. The median follow-up of the entire cohort was 41 months. The baseline information of the entire cohort as stratified by age is shown in Table 1. With advancing age, an increasing proportion of patients underwent ablation rather than resection (age 65 - 69, 44.6%; age 70 - 74, 56.0%; age ≥ 75, 65.1%, P < 0.001). Significantly more elderly patients had comorbid diseases (hypertension, P < 0.001; heart disease, P = 0.002) but fewer had hepatitis B infection (P = 0.001). Multivariate analyses revealed that the type of treatment (resection vs. ablation), tumor number, AFP level, serum albumin level and diabetes were independent factors associated with OS, and the type of treatment (resection vs. ablation), tumor number and AFP level were independent factors associated with RFS (Supplementary Material 1, www.wjon.org).
 Click to view | Table 1. Baseline Information of Patients Stratified by Age |
Impact of treatment on survival associated with age
The baseline information of the three age subgroups of patients as stratified by treatment type after IPTW and before IPTW is shown in Table 2 and Supplementary Material 2 (www.wjon.org), respectively. In the subgroups of patients aged 65 - 69 and 70 - 74 years, liver resection resulted in improved survival outcomes compared with those of ablation. The gap in OS outcomes between these two types of treatment narrowed with increasing age (resection vs. ablation, for age 65 - 69, P < 0.001, HR = 0.38, 95% CI: 0.29 - 0.50; for age 70 - 74, P = 0.012, HR = 0.64). Furthermore, for the subgroup of patients aged ≥ 75 years, ablation resulted in a similar OS as resection (resection vs. ablation, HR = 0.84, 95% CI: 0.55 - 1.30, P = 0.44) (Fig. 1). This observation was supported by our findings that there was a significant interactive effect of age and treatment on OS (Table 3). However, patients who underwent resection had a better RFS than those who underwent ablation in all age subgroups (Supplementary Material 3, www.wjon.org, www.wjon.org). No interactive effect of age and treatment was found on RFS (Table 3).
 Click to view | Table 2. Baseline Information of Patients Stratified by Age and Treatment Type After IPTW |
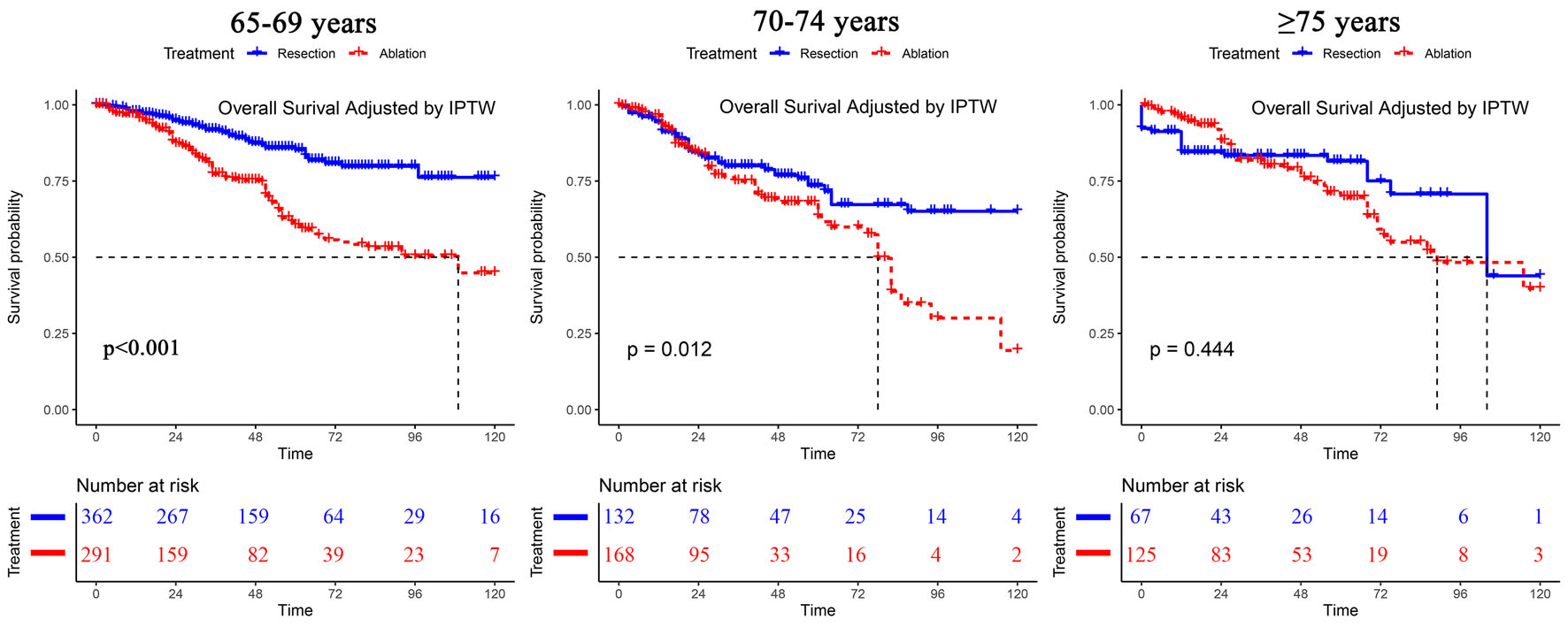 Click for large image | Figure 1. Overall survival analyses of patients stratified by age after IPTW. IPTW: inverse probability of treatment weighting. |
 Click to view | Table 3. Effect of Treatment on Survival Across Age Groups |
Subgroup analyses in patients aged 65 - 69 and 70 - 74 years as stratified by tumor size
Among the subgroup of patients aged 65 - 69 years with a single tumor of 0 - 20 mm, the OS was similar after ablation and liver resection (resection vs. ablation, HR = 0.66, 95% CI: 0.34 - 1.27, P = 0.214). Likewise, for the subgroup of patients aged 70 - 74 with a single tumor of 0 - 20 mm, ablation resulted in a similar OS as resection (resection vs. ablation, HR = 1.49, 95% CI: 0.59 - 3.78, P = 0.403). However, with increased tumor size (21 - 30 mm), the results differed significantly between the subgroup of patients aged 65 - 69 and those aged 70 - 74. Among the subgroup of patients aged 65 - 69 with a single tumor of 21 - 30 mm, resection resulted in significantly better OS than ablation (resection vs. ablation, HR = 0.35, 95% CI: 0.19 - 0.64, P = 0.001). However, in the subgroup of patients who were aged 70 - 74 with a single tumor of 21 - 30 mm, ablation resulted in similar OS as resection (resection vs. ablation, HR = 0.65, 95% CI: 0.25 - 1.75, P = 0.397) (Fig. 2). The survival analyses of RFS are shown in Supplementary Material 4-6 (www.wjon.org).
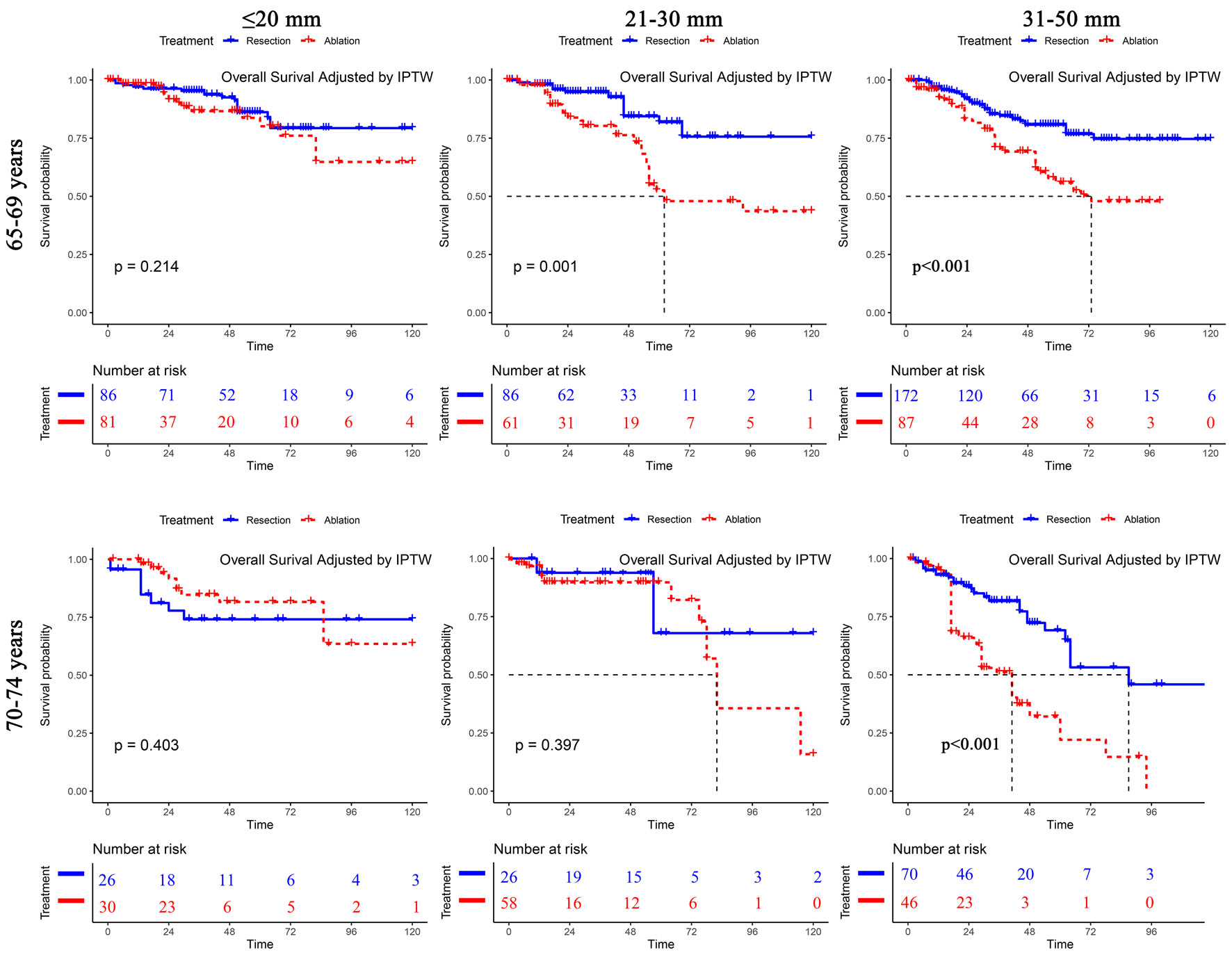 Click for large image | Figure 2. Subgroup overall survival analyses of patients with a single tumor stratified by tumor size among different age groups after IPTW. IPTW: inverse probability of treatment weighting. |
Causes of death across the age subgroups
Compared with the age 65 - 69 subgroup, the age 70 - 74 and age ≥ 75 subgroups had significantly lower rates of HCC-associated mortality but significantly higher mortality attributed to liver-associated comorbidities and other causes (Table 1).
Comparison of other related factors
Although ablation was associated with an increased number of hospitalizations for the treatment of HCC, no significant differences were found in total in-hospital stay and total cost for HCC treatment between the ablation and resection groups. Resection was associated with a longer treatment time than ablation. Patients who underwent resection had a significantly higher rate of intensive care unit admission than patients who underwent ablation (Table 4).
 Click to view | Table 4. Comparison of Other Related Events Between the Resection and Ablation Group After IPTW |
| Discussion | ▴Top |
This study compared the long-term survival outcomes between hepatic resection and ablation in elderly patients with very-early/early stages of HCC. The treatment outcomes of percutaneous ablation approached those achieved by hepatic resection with increasing patient age. We found that the HCC-related death rate was higher in patients aged 65 - 69, and the liver/other cause-related death rate was higher in patients aged > 69. Among patients who were aged ≥ 75 with very-early/early stages of HCC of ≤ 50 mm in size, ablation achieved similar OS outcomes as resection. Further subgroup analyses indicated that for patients aged 65 - 69 with a single tumor of 0 - 20 mm and for those aged 70 - 74 with a single tumor of 0 - 30 mm, ablation resulted in a similar OS to that of resection.
Although many studies have compared the treatment outcomes of hepatic resection with those of ablation for HCC [9-11, 17-26], very few studies have been conducted on elderly patients. In the study by Kaibori and associates [7] based on data on elderly patients with HCC in Japan, the survival outcomes of hepatic resection (n = 750) and ablation (n = 1,562) in patients with HCC with lesions smaller than 3 cm were compared using propensity score matching (PSM). The authors concluded that hepatic resection resulted in a significantly decreased risk of HCC recurrence and improved OS. This study, however, failed to compare the treatment outcomes of hepatic resection and ablation for HCCs 0 - 2 cm. In contrast, Peng and associates [8] compared the treatment outcomes of hepatic resection with RFA in elderly patients and concluded that RFA resulted in better survival for patients with lesions less than 3 cm than hepatic resection. This conclusion contradicted the results obtained by Kaibori and associates. In the Peng et al’s study, the main limitation was the small sample sizes of the two treatment groups, with 91 patients in the hepatic resection group and 89 patients in the RFA group.
In our study, we used IPTW for all the comparative analyses, including subgroup analyses, to minimize bias. For patients with a single tumor ≤ 5 cm or no more than three tumors ≤ 3 cm each, our study found improved survival outcomes after percutaneous ablation compared with hepatic resection with increasing patient age. When the patient age subgroup was ≥ 75, the long-term survival outcomes after ablation were similar to those after liver resection. As hepatic resection is more invasive than ablation, the benefits resulting from liver resection decrease with aging. This phenomenon is in line with the results obtained by Cucchetti and associates [27] that with aging, the oldest group of patients with HCC who underwent liver resection had the shortest overall postoperative lifespan and the smallest number of years of life lost. In our current study, the causes of death across the several age subgroups showed age to be associated with significantly more deaths caused by non-HCC-associated causes. This result is consistent with that of the study by Kaibori and associates [28], which demonstrated that the cumulative incidences of other causes of death in elderly patients were significantly different from those of HCC-associated deaths. However, another study by Kaibori and associates [7] demonstrated that resection still resulted in better OS in patients aged ≥ 75 years with a primary HCC ≤ 3.0 cm. In our opinion, it would be difficult to reach a solid conclusion on whether resection or ablation can provide better survival outcomes in this elderly population of patients. However, based on the interactive effect of age and treatment found in our study, patients with increasing age seemed to benefit similarly from percutaneous ablation as from hepatic resection. Although patients who underwent ablation had worse RFS than those who underwent liver resection, control of HCC was still achievable by performing repeated ablations, which seems to be a reasonable treatment strategy for these elderly patients.
To further compare the treatment outcomes between hepatic resection and ablation in the subgroups of different tumor sizes in our study, patients with a single tumor were further subdivided into a 0 to 20 mm subgroup, a 21 to 30 mm subgroup and a 31 to 50 mm subgroup. In the age 65 - 74 years subgroup with a 0 to 20 mm tumor, there was no significant difference in OS between the two treatments. In the 65- to 69-year-old subgroup with a 21- to 30-mm tumor, hepatic resection resulted in significantly better OS than ablation. In the 70- to 74-year-old subgroup, ablation resulted in a similar OS to that of liver resection. The highlight of the current study is that, unlike previous studies that focused only on elderly patients in different age subgroups, this study further subdivided tumors less than 30 mm in size into 0 to 20 mm and 21 to 30 mm subgroups. The difference in the results between this study and the study by Kaibori and associates can be explained by the latter study, which did not separate the two groups of 0 to 20 mm and 21 to 30 mm into two subgroups.
This study has limitations. First, data on postoperative complications were not collected, resulting in no comparative analysis of postoperative complications. Second, the data on antiviral treatment of the patients in this study were not known in a significant proportion of patients; thus, this factor could not be analyzed. Antiviral treatment affects the long-term survival of HCC patients after treatment with curative intention.
Conclusion
In conclusion, the results of this study indicated that there was a significant interactive effect of age and treatment on long-term survival outcomes in HCC patients. With increasing age, the treatment outcomes of ablation become similar to those of resection. A higher liver/other cause-related death rate in very elderly patients may shorten their life expectancy, which may lead to the same OS regardless of whether resection or ablation is chosen.
| Supplementary Material | ▴Top |
Suppl 1. Multivariate analysis for overall survival and recurrence-free survival.
Suppl 2. The baseline information of the three age groups stratified by treatment type before IPTW.
Suppl 3. OS survival analyses of patients stratified by age and treatment type before IPTW.
Suppl 4. Subgroup OS analyses of patients with a single tumor stratified by tumor size and treatment type among different age groups before IPTW.
Suppl 5. RFS analyses of patients stratified by age and treatment type before and after IPTW.
Suppl 6. Subgroup RFS analyses of patients with a single tumor stratified by tumor size and treatment type among different age groups before and after IPTW.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by the National Natural Science Foundation of China-Guangdong Joint Fund (U20A20370), the Guangzhou Science and Technology Program, Key Projects of Collaborative Innovation of Health Medicine (No. 201704020228); Guangzhou Science and Technology Program, China Key Projects of Collaborative Innovation of Production, Learning and Research (No. 201704020134); National Natural Science Foundation of China (No. 81771955, 81702393); Natural Science Foundation of Guangdong Province, 2017A030310373; Sun Yat-sen University Clinical Trial 5010 Project (No. 2016002).
Conflict of Interest
All authors declare that they have no conflict of interest related to this manuscript.
Informed Consent
Written informed consent was obtained from each subject.
Author Contributions
Hong Liang Zou made primary contributions to the conception and design, acquisition of data, and analysis and interpretation of data and participated in critical drafting and revising of the article for important intellectual content. Chao An, Lu Jun Shen and Hui Tang made substantial contributions to the conception and design and acquisition of data. Ji Bin Li made contributions to the design and acquisition of data. Wan Yee Lau critically drafted the final version of the article. Yi Quan Jiang and Jin Hua Huang gave final approval of the version to be submitted and any revised versions.
Data Availability
The authenticity of this article was validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number of RDDA2019001326. The datasets generated and/or analyzed during the current study are not publicly available but are available from the corresponding author upon reasonable request.
Abbreviations
HCC: hepatocellular carcinoma; RFA: radiofrequency ablation; MWA: microwave ablation; IPTW: inverse probability of treatment weighting; OS: overall survival; RFS: recurrence-free survival; HR: hazard ratio; CI: confidence interval; AFP: α-fetoprotein; ALT: alanine aminotransferase; AST: aspartate aminotransferase; PLT: platelet; PT: prothrombin time; INR: international normalized ratio; ALB: albumin; TBIL: total bilirubin
| References | ▴Top |
- Villanueva A. Hepatocellular carcinoma. N Engl J Med. 2019;380(15):1450-1462.
doi - Forner A, Reig M, Bruix J. Hepatocellular carcinoma. Lancet. 2018;391(10127):1301-1314.
doi - Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394-424.
doi - Cho E, Cho HA, Jun CH, Kim HJ, Cho SB, Choi SK. A review of hepatocellular carcinoma in elderly patients focused on management and outcomes. In Vivo. 2019;33(5):1411-1420.
doi - Chen RC, Royce TJ, Extermann M, Reeve BB. Impact of age and comorbidity on treatment and outcomes in elderly cancer patients. Semin Radiat Oncol. 2012;22(4):265-271.
doi - Daste A, Domblides C, Gross-Goupil M, Chakiba C, Quivy A, Cochin V, de Mones E, et al. Immune checkpoint inhibitors and elderly people: A review. Eur J Cancer. 2017;82:155-166.
doi - Kaibori M, Yoshii K, Hasegawa K, Ogawa A, Kubo S, Tateishi R, Izumi N, et al. Treatment optimization for hepatocellular carcinoma in elderly patients in a Japanese nationwide cohort. Ann Surg. 2019;270(1):121-130.
doi - Peng ZW, Liu FR, Ye S, Xu L, Zhang YJ, Liang HH, Lin XJ, et al. Radiofrequency ablation versus open hepatic resection for elderly patients (> 65 years) with very early or early hepatocellular carcinoma. Cancer. 2013;119(21):3812-3820.
doi - Cucchetti A, Piscaglia F, Cescon M, Colecchia A, Ercolani G, Bolondi L, Pinna AD. Cost-effectiveness of hepatic resection versus percutaneous radiofrequency ablation for early hepatocellular carcinoma. J Hepatol. 2013;59(2):300-307.
doi - Huang G, Chen X, Lau WY, Shen F, Wang RY, Yuan SX, Geng WX, et al. Quality of life after surgical resection compared with radiofrequency ablation for small hepatocellular carcinomas. Br J Surg. 2014;101(8):1006-1015.
doi - Kang TW, Kim JM, Rhim H, Lee MW, Kim YS, Lim HK, Choi D, et al. Small hepatocellular carcinoma: radiofrequency ablation versus nonanatomic resection—propensity score analyses of long-term outcomes. Radiology. 2015;275(3):908-919.
doi - Mirici-Cappa F, Gramenzi A, Santi V, Zambruni A, Di Micoli A, Frigerio M, Maraldi F, et al. Treatments for hepatocellular carcinoma in elderly patients are as effective as in younger patients: a 20-year multicentre experience. Gut. 2010;59(3):387-396.
doi - Yu B, Ding Y, Liao X, Wang C, Wang B, Chen X. Radiofrequency ablation versus surgical resection in elderly patients with early-stage hepatocellular carcinoma in the era of organ shortage. Saudi J Gastroenterol. 2018;24(6):317-325.
doi - Makuuchi M, Hasegawa H, Yamazaki S. Ultrasonically guided subsegmentectomy. Surg Gynecol Obstet. 1985;161(4):346-350.
- de Lope CR, Tremosini S, Forner A, Reig M, Bruix J. Management of HCC. J Hepatol. 2012;56(Suppl 1):S75-87.
doi - Livraghi T, Meloni F, Di Stasi M, Rolle E, Solbiati L, Tinelli C, Rossi S. Sustained complete response and complications rates after radiofrequency ablation of very early hepatocellular carcinoma in cirrhosis: Is resection still the treatment of choice? Hepatology. 2008;47(1):82-89.
doi - Liu PH, Hsu CY, Hsia CY, Lee YH, Huang YH, Chiou YY, Lin HC, et al. Surgical resection versus radiofrequency ablation for single hepatocellular carcinoma </= 2 cm in a propensity score model. Ann Surg. 2016;263(3):538-545.
doi - Chen MS, Li JQ, Zheng Y, Guo RP, Liang HH, Zhang YQ, Lin XJ, et al. A prospective randomized trial comparing percutaneous local ablative therapy and partial hepatectomy for small hepatocellular carcinoma. Ann Surg. 2006;243(3):321-328.
doi - Cho YK, Kim JK, Kim MY, Rhim H, Han JK. Systematic review of randomized trials for hepatocellular carcinoma treated with percutaneous ablation therapies. Hepatology. 2009;49(2):453-459.
doi - Fang Y, Chen W, Liang X, Li D, Lou H, Chen R, Wang K, et al. Comparison of long-term effectiveness and complications of radiofrequency ablation with hepatectomy for small hepatocellular carcinoma. J Gastroenterol Hepatol. 2014;29(1):193-200.
doi - Feng K, Yan J, Li X, Xia F, Ma K, Wang S, Bie P, et al. A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol. 2012;57(4):794-802.
doi - Gory I, Fink M, Bell S, Gow P, Nicoll A, Knight V, Dev A, et al. Radiofrequency ablation versus resection for the treatment of early stage hepatocellular carcinoma: a multicenter Australian study. Scand J Gastroenterol. 2015;50(5):567-576.
doi - Huang J, Yan L, Cheng Z, Wu H, Du L, Wang J, Xu Y, et al. A randomized trial comparing radiofrequency ablation and surgical resection for HCC conforming to the Milan criteria. Ann Surg. 2010;252(6):903-912.
doi - Kutlu OC, Chan JA, Aloia TA, Chun YS, Kaseb AO, Passot G, Yamashita S, et al. Comparative effectiveness of first-line radiofrequency ablation versus surgical resection and transplantation for patients with early hepatocellular carcinoma. Cancer. 2017;123(10):1817-1827.
doi - Lee HW, Lee JM, Yoon JH, Kim YJ, Park JW, Park SJ, Kim SH, et al. A prospective randomized study comparing radiofrequency ablation and hepatic resection for hepatocellular carcinoma. Ann Surg Treat Res. 2018;94(2):74-82.
doi - Miura JT, Johnston FM, Tsai S, Eastwood D, Banerjee A, Christians KK, Turaga KK, et al. Surgical resection versus ablation for hepatocellular carcinoma </= 3 cm: a population-based analysis. HPB (Oxford). 2015;17(10):896-901.
doi - Cucchetti A, Sposito C, Pinna AD, Citterio D, Ercolani G, Flores M, Cescon M, et al. Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg. 2016;103(2):e93-99.
doi - Kaibori M, Yoshii K, Yokota I, Hasegawa K, Nagashima F, Kubo S, Kon M, et al. Impact of advanced age on survival in patients undergoing resection of hepatocellular carcinoma: report of a Japanese nationwide survey. Ann Surg. 2019;269(4):692-699.
doi
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


