| World Journal of Oncology, ISSN 1920-4531 print, 1920-454X online, Open Access |
| Article copyright, the authors; Journal compilation copyright, World J Oncol and Elmer Press Inc |
| Journal website https://www.wjon.org |
Letter to the Editor
Volume 13, Number 5, October 2022, pages 320-324
Precise Therapeutic Benefits and Underlying Mechanisms of Anlotinib Combined With Checkpoint Immunotherapy in Advanced Non-Small-Cell Lung Cancer
Yi Xianga, Xiao Juan Lub, Yi Hang Sunc, Xia Liua, Yan Yang Wud, Hui Raod, Qiu Yang Qued, Qi Xiaoe, Rui Zhua, Xiao Fei Fenga, Wei Ling Laia, Xiao Huan Zouf, Ying Wangd, Song Qiud, Li Huanga, g, h, Hua Qiu Shia, g, h, Xiang Cai Wanga, g, h
aDepartment of Oncology, The First Affiliated Hospital, Gannan Medical University, Ganzhou, China
bDepartment of Oncology, The People’s Hospital of Leshan, Leshan, China
cSchool of Pharmaceutical Sciences, Southern Medical University, Guangzhou, China
dFirst Clinical Medical College, Gannan Medical University, Ganzhou, China
eJiangkou Town Central Health Center, Ganzhou, China
fDepartment of Critical Care Medicine, The First Affiliated Hospital, Gannan Medical University, Ganzhou, China
gThese authors contributed equally to this work.
hCorresponding Author: Li Huang, Hua Qiu Shi, and Xiang Cai Wang, Department of Oncology, The First Affiliated Hospital, Gannan Medical University, Ganzhou, China;;
Manuscript submitted May 12, 2022, accepted August 6, 2022, published online October 22, 2022
Short title: Anlotinib Combined With ICIs in NSCLC
doi: https://doi.org/10.14740/wjon1494
| To the Editor | ▴Top |
Despite the wide clinical application of immune checkpoint inhibitors (ICIs) monotherapy in advanced non-small-cell lung cancer (NSCLC), the limited benefit rate still puzzles researchers. One of the mechanisms of immunotherapy resistance may be an abnormal vascular system in the immunosuppressive tumor microenvironment (TME), which can inhibit dendritic cell maturation and prevents T-cell invasion into tumors [1, 2]. Previous studies have shown that anti-angiogenesis therapy modulates the immunosuppressive TME and may help reverse ICIs resistance [3, 4], suggesting potential clinical benefits of combined anti-angiogenesis and checkpoint blockade [5]. In fact, the combination of small-molecule tyrosine kinase inhibitors (TKIs) and ICIs was not just proposed recently. Early phase clinical trials have started to evaluate the immune-based combination strategy as a front-line or posterior-line therapy in patients with such as renal and liver cancer [6, 7]. These encouraging results underscore the need for further research into the therapeutic response to and underlying mechanisms of the combination of these two standard therapies in the post-first-line treatment for patients with advanced NSCLC.
In this study, we surveyed the efficacy and safety of anlotinib, a newly developed oral multi-target small-molecule anti-angiogenesis TKI, combined with programmed death 1 (PD-1) mAb in patients with advanced NSCLC, using a retrospective method based on real-world evidence (Supplementary Materials and Methods). We then investigated anlotinib-induced biological responses in the NSCLC immune microenvironment and their predictive performance for patient prognosis and immunotherapy outcomes using bioinformatics analysis. These results showed that their synergetic effect has good clinical application prospects and is worthy of further study.
Between July 2018 and October 2021, 142 patients, 106 men (74.6%) and 36 women (25.4%), aged 37 - 81 years (median age 59), with advanced NSCLC were enrolled in this retrospective clinical study. Baseline demographics and clinicopathological characteristics of these patients are summarized in Supplementary Material 7 (www.wjon.org). A total of 128 patients (90.1%) were diagnosed with stage IV disease, and almost half (72 patients, 50.7%) had ≥ 3 metastatic sites. Ninety patients (63.4%) were diagnosed with adenocarcinomas. There was no significant selection bias.
We observed and analyzed the median progression-free survival (mPFS), median overall survival (mOS), overall response rate (ORR), disease control rate, and incidence of adverse events in the different treatment groups. As shown in the histogram (Fig. 1a) and Supplementary Material 8 (www.wjon.org), 23 patients who received a combination of anlotinib and PD-1 mAb (A+P) therapy achieved PR, showing significant improvement over the monotherapy groups. Survival analyses indicating the mPFS of all 142 patients was 5.10 months, and the mOS was 9.02 months (Fig. 1b). Individually, the mPFS was 4.07 months in anlotinib group, 5.13 months in PD-1 mAb group and 7.13 months in the A+P combination group. The mOS of anlotinib, PD-1 mAb, and A+P group were 6.23, 8.79, and 14.01 months, respectively (Fig. 1c). The A+P combination group showed a significantly better PFS and OS than the other two treatment groups (Supplementary Material 9, 10, www.wjon.org). Besides, subgroup analysis showed that patients with earlier TNM stage, fewer than three metastatic sites or previous less than third-line treatment had better clinical outcomes (Supplementary Material 1, www.wjon.org). In summary, the difference in patient response suggests the superiority of combination therapy over monotherapy.
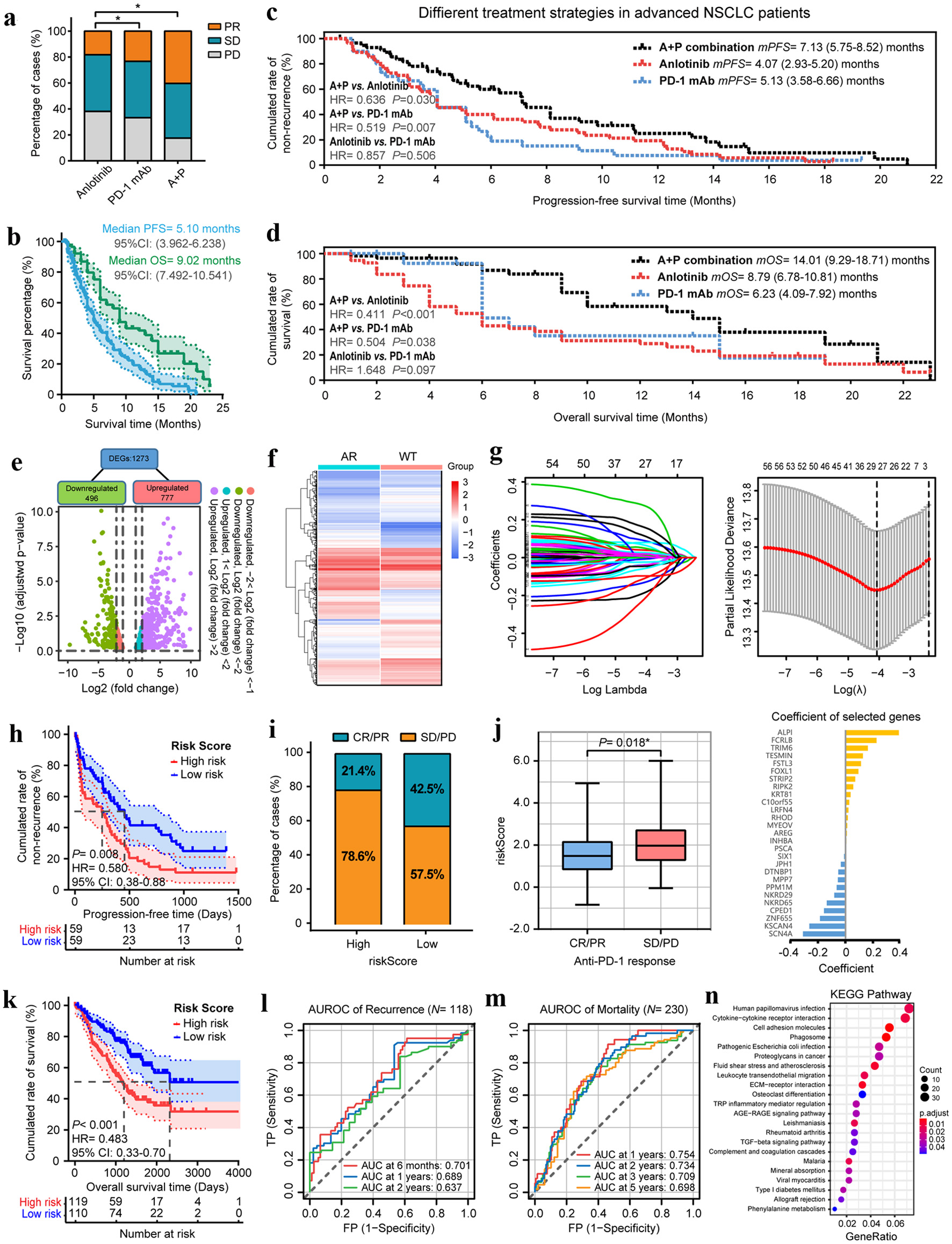 Click for large image | Figure 1. Anlotinib changes the TME and broadens the benefits of anti-PD-1 immunotherapy in patients with advanced NSCLC. (a) The objective tumor response of patients in anlotinib, PD-1 mAb and A+P combination groups. (b) The PFS and OS curves of overall patients. (c) The PFS and OS curves of patients in anlotinib, PD-1 mAb and A+P combination groups. (d) Scatter-plot of anlotinib-responsive gene differential screening. (e) Heatmap of the differential expressions of 1,273 DEGs after anlotinib treatments. (f) Coefficients of LASSO regression analysis. (g) The cross-validation results of LASSO regression and coefficients of selected genes. (h) Kaplan-Meier analysis demonstrated that the PFS of patients who received PD-1 mAb in the high-risk group were significantly shorter than those of the low-risk group. (i) The ORR of patients in high- and low-risk groups. (j) Box-plot of the ARIM-scores for different anti-tumor treatment responses. (k) The OS of patients who received PD-1 mAb in the high- and low-risk groups. (l, m) Time-dependent ROC curves for recurrence and mortality of patients who received PD-1 mAb at different follow-up times. (n) KEGG pathway enrichment analysis of the 1273 DEGs. *P < 0.05, **P < 0.01. |
Subsequently, treatment-related adverse events (TRAEs) that occurred in the different groups were collected and listed in Supplementary Material 11 (www.wjon.org). The main TRAEs were fatigue (53, 37.3%), anorexia (45, 40.9%) and headache/dizziness (47, 33.1%). The less common TRAEs were vomiting (11, 7.7%), dyspnea (5, 3.5%), and hemoptysis (9, 6.3%). The A+P combination treatment can be considered safe, with no significant increase in toxicity load compared with monotherapy.
We further explored the influence of anlotinib on ICIs immunotherapy outcomes and changes in TME using bioinformatics analysis. By downloading the GSE142031 dataset from the gene expression omnibus (GEO) database [8], a total of 1,273 differentially expressed genes (DEGs) were identified between the anlotinib-resistant and anlotinib-responsive NCI-H1975 lung cell lines (Fig. 1d, e). Using select NSCLC samples in TCGA database (Supplementary Material 12, 13, www.wjon.org), 57 prognostic genes were identified and then screened via univariate Cox regression analysis by setting P-value < 0.01. Forest-plots for the top 20 prognostic genes are shown in Supplementary Material 2A (www.wjon.org), and Kaplan-Meier curve exhibited that the top three genes, ANKRD65, FSTL3, and LRIG1, were closely related to patient survival (Supplementary Material 2B-D, www.wjon.org).
After further analysis using least absolute shrinkage and selection operator (LASSO) regression and multivariate Cox regression [9] (Figure 1f, g and Supplementary Material 14, www.wjon.org), 13 genes were selected to assemble an anlotinib-responsive immune-microenvironment risk-score (ARIM-score). Patients were divided into high-risk and low-risk groups, and the median risk score was used as the threshold. The results demonstrated that the OS of patients in the low-risk group was longer than that of the high-risk group (Supplementary Materials 2E-G, 3, www.wjon.org).
Based on the short-term and long-term analyses of five NSCLC immunotherapy datasets downloaded from the GEO database, we found that the modification of the lung TME by anlotinib may play a role in promoting the therapeutic effect of anti-PD-1 mAb. Figure 1h, k shows that the PFS and OS of the high-risk group were significantly shorter than those of the low-risk group. The ORR of the high-and low-risk groups were 21.4% and 42.5%, respectively (Fig. 1i). Patients who achieved an objective response also had a lower risk score than those who did not respond to treatment (Fig. 1j). Ultimately, the ROC curves and the results of AUCs in Figure 1l, M indicated that the ARIM-score was a promising prognostic indicator for mortality and recurrence in patients with NSCLC who received anti-PD-1 immunotherapy.
The correlation between the ARIM-score and the sensitivity of different antitumor agents in the CGP database [10] was evaluated using the R-package pRRophetic (Supplementary Material 4A, www.wjon.org). Furthermore, univariate and multivariate Cox regression analyses showed that the ARIM-score, patient age, and TNM stage were independent prognostic factors for patients with NSCLC (Supplementary Material 4B, C and Supplementary Material 15, 16, www.wjon.org). We constructed nomograms to guide clinical practice based on these characteristics (Supplementary Material 5, www.wjon.org).
At last, the biological classification and characterization of the 1,273 DEGs were performed for exploring the underlying mechanisms of anlotinib in regulating the therapeutic benefits of immunotherapy. Results of the KEGG pathway analysis revealed that the DEGs were mainly enriched in cytokine receptor interaction, cell adhesion molecules, phagosome and leukocyte trans-endothelial migration signaling pathways (Fig. 1n). Extracellular matrix structural constitutes and glycosaminoglycan binding were found to be the dominant cell functions identified in the GO analysis (Supplementary Material 6 and Supplementary Material 17, www.wjon.org).
For many years, anlotinib was approved as a third-line treatment in advanced NSCLC. Nevertheless, there have been critical innovations in the previous treatment modalities during a relatively short period of time. Many studies have found that many small-molecule TKIs, such as anlotinib in this study, can participate in the regulation of anti-PD-1 immunotherapy in multiple cancer types, in addition to their previously identified functions [5]. As described earlier in this letter, a robust biological theory supported the evaluation in immunotherapy + TKIs, while our clinical data and other early clinical trials suggest the combination of TKIs with immunotherapy. Our findings showed reliable efficacy and tolerability of anlotinib and anti-PD-1 mAb combination therapy in patients with advanced NSCLC as a post-first-line treatment. Bioinformatics analysis results demonstrated that anlotinib changes the TME and broadens the benefits of anti-PD-1 immunotherapy. The A+P combination might transform the immunosuppressive setting into another “immunosupportive” setting, thereby improving clinical outcomes and responses. The promising results of anlotinib with immunotherapy, as well as other immune-based combinations, might also expand the therapeutic armamentarium against advanced NSCLC in the near future [6]. In this setting, further efforts should be focused on the identification of biomarkers predictive of response to immune-based combinations for guiding future treatment decision-making on the basis of clinical benefits in patients with advanced disease. Through analyses of GEO and TCGA clinical samples, the key DEGs were identified and used to construct the ARIM-score, a predictive model for patient survival and immunotherapy outcomes in NSCLC, which will lay a foundation for the development of new vascular endothelial growth factor (VEGF)/vascular endothelial growth factor receptor 2 (VEGFR2) and PD-1 pathway inhibition therapies.
| Supplementary Material | ▴Top |
Suppl 1. (A) Flow chart of patient enrollment and study procedure. The PFS (B) and OS (C) curves of patients in different TNM stages. The PFS (D) and OS (E) curves of patients with different number of metastatic sites. The PFS (F) and OS (G) curves of patients who received different number of previous treatments.
Suppl 2. (A) Forest-plot for the top 20 prognostic genes identified by univariate Cox analysis. Kaplan-Meier analyses of the top three genes, ANKRD65 (B), FSTL3 (C), and LRIG1 (D), in patients with NSCLC. (E) The OS of patients in the high- and low-risk groups, divided by using the median ARIM-score as the threshold. (F) Scatter-plot of the ARIM-scores. (G) ROC curves showing the efficacy of the ARIM-score on 1-year, 3-year and 5-year OS.
Suppl 3. The prognostic performance of the ARIM-score in several subgroups: different pathologic types (A-B), TNM stages (C-D), and EGFR mutation status (E-F).
Suppl 4. (A) Box-plots showed the IC50 of different anti-tumor agents in the CGP databases for patients with high or low ARIM-scores. Univariate (B) and multivariate (C) Cox regression analyses showed that the ARIM-score, patient age, and TNM stage were independent prognostic factors for patients with NSCLC.
Suppl 5. The nomograms constructed based on the characteristics for guiding clinical practice.
Suppl 6. GO enrichment analysis of the 1,273 DEGs.
Suppl 7. Correlation between different treatment strategies and clinicopathological characteristics in advanced NSCLC patients.
Suppl 8. Clinical efficacy of anlotinib combined with anti-PD-1 Immunotherapy in advanced NSCLC patients.
Suppl 9. Univariate and multivariate Cox regression analysis for recurrence in advanced NSCLC patients. Abbreviations: HR: hazard ratio; CI: confidence interval. *P < 0.05, **P < 0.01, ***P < 0.001.
Suppl 10. Univariate and multivariate Cox regression analysis for mortality in advanced NSCLC patients.
Suppl 11. Treatment-Related Adverse Events.
Suppl 12. The expression profile dataset in advanced NSCLC patients downloaded from online databases.
Suppl 13. Patient and clinical characteristics of TCGA dataset.
Suppl 14. Anlotinib-responsive markers.
Suppl 15. Univariate Cox regression analysis of anlotinib-respond markers.
Suppl 16. Multivariate Cox regression analysis of anlotinib-respond markers.
Suppl 17. GO and KEGG pathway enrichment analysis of anlotinib-respond markers.
Acknowledgments
None to declare.
Financial Disclosure
This study was supported by the Scientific Research Foundation of Gannan Medical University (2021DQ095 to Yi Xiang), and Beijing Medical and Health Foundation (B183017).
Conflict of Interest
The authors have declared that no competing interest exists.
Author Contributions
Yi Xiang designed the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Xiao Jun Lu, and Ying Wang performed the database analysis from TCGA and GEO. Song Qiu performed the clinical data analysis from NSCLC patients. Cox and LASSO regression were carried out by Yi Hang Sun and Xia Liu. Yan Yang Wu, Hui Rao and Qiu Yang Que did the construction and validation of prognostic models. Yi Xiang, Qi Xiao and Rui Zhu wrote the manuscript, and Xiao Fei Feng, Wei Ling Lai and Xiao Huan Zou revised the manuscript. All these works were planned and supervised by Li Huang, Hua Qiu Shi and Xiang Cai Wang. All authors have read and approved the final manuscript.
Data Availability
The bioinformatics analysis data utilized in this study are publicly available and listed below: the GEO database (https://www.ncbi.nlm.nih.gov/geo/); TCGA (https://cancergenome.nih.gov). And the clinical and prognostic data generated and analyzed during the current study are available in supplementary materials or from the corresponding author on reasonable request.
Abbreviations
NSCLC: non-small-cell lung cancer; TME: tumor microenvironment; PD-1: programmed death 1; ECOG PS: Eastern Cooperative Oncology Group performance status; VEGFR: vascular epithelial growth factor receptor; VEGF: vascular endothelial growth factor; CR: complete response; PR: partial response; SD: stable disease; PD: progressive disease; ORR: overall response rate; PFS: progression-free survival; OS: overall survival; HR: hazard ratio; CI: confidence interval; ROC: receiver operating characteristic; AUC: area under the curve; TRAEs: treatment-related adverse events; TCGA: The Cancer Genome Atlas; GEO: gene expression omnibus; DEG: differentially expressed gene; LASSO: least absolute shrinkage and selection operator; ARIM-score: anlotinib-responsive immune microenvironment risk-score; CGP: Cancer Genome Project
| References | ▴Top |
- Kopecka J, Salaroglio IC, Perez-Ruiz E, Sarmento-Ribeiro AB, Saponara S, De Las Rivas J, Riganti C. Hypoxia as a driver of resistance to immunotherapy. Drug Resist Updat. 2021;59:100787.
doi pubmed - Tian L, Goldstein A, Wang H, Ching Lo H, Sun Kim I, Welte T, Sheng K, et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature. 2017;544(7649):250-254.
doi pubmed - Huinen ZR, Huijbers EJM, van Beijnum JR, Nowak-Sliwinska P, Griffioen AW. Anti-angiogenic agents - overcoming tumour endothelial cell anergy and improving immunotherapy outcomes. Nat Rev Clin Oncol. 2021;18(8):527-540.
doi pubmed - Georganaki M, van Hooren L, Dimberg A. Vascular Targeting to Increase the Efficiency of Immune Checkpoint Blockade in Cancer. Front Immunol. 2018;9:3081.
doi pubmed - Yi M, Jiao D, Qin S, Chu Q, Wu K, Li A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol Cancer. 2019;18(1):60.
doi pubmed - Rizzo A, Dadduzio V, Ricci AD, Massari F, Di Federico A, Gadaleta-Caldarola G, Brandi G. Lenvatinib plus pembrolizumab: the next frontier for the treatment of hepatocellular carcinoma? Expert Opin Investig Drugs. 2022;31(4):371-378.
doi pubmed - Rizzo A, Mollica V, Santoni M, Ricci AD, Rosellini M, Marchetti A, Montironi R, et al. Impact of clinicopathological features on survival in patients treated with first-line immune checkpoint inhibitors plus tyrosine kinase inhibitors for renal cell carcinoma: a meta-analysis of randomized clinical trials. Eur Urol Focus. 2022;8(2):514-521.
doi pubmed - Zhang LL, Lu J, Liu RQ, Hu MJ, Zhao YM, Tan S, Wang SY, et al. Chromatin accessibility analysis reveals that TFAP2A promotes angiogenesis in acquired resistance to anlotinib in lung cancer cells. Acta Pharmacol Sin. 2020;41(10):1357-1365.
doi pubmed - Klement RJ, Belderbos J, Grills I, Werner-Wasik M, Hope A, Giuliani M, Ye H, et al. Prediction of early death in patients with early-stage NSCLC-can we select patients without a potential benefit of SBRT as a curative treatment approach? J Thorac Oncol. 2016;11(7):1132-1139.
doi pubmed - Forbes SA, Bindal N, Bamford S, Cole C, Kok CY, Beare D, Jia M, et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 2011;39(Database issue):D945-D950.
doi pubmed
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial 4.0 International License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
World Journal of Oncology is published by Elmer Press Inc.


